当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acceleration of CO2 insertion into metal hydrides: ligand, Lewis acid, and solvent effects on reaction kinetics†
Chemical Science ( IF 8.4 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1039/c8sc02535e Jessica E Heimann 1 , Wesley H Bernskoetter 2 , Nilay Hazari 1 , James M Mayer 1
Chemical Science ( IF 8.4 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1039/c8sc02535e Jessica E Heimann 1 , Wesley H Bernskoetter 2 , Nilay Hazari 1 , James M Mayer 1
Affiliation
|
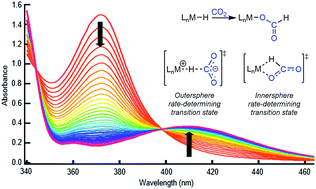
The insertion of CO2 into metal hydrides and the microscopic reverse decarboxylation of metal formates are important elementary steps in catalytic cycles for both CO2 hydrogenation to formic acid and methanol as well as formic acid and methanol dehydrogenation. Here, we use rapid mixing stopped-flow techniques to study the kinetics and mechanism of CO2 insertion into transition metal hydrides. The investigation finds that the most effective method to accelerate the rate of CO2 insertion into a metal hydride can be dependent on the nature of the rate-determining transition state (TS). We demonstrate that for an innersphere CO2 insertion reaction, which is proposed to have a direct interaction between CO2 and the metal in the rate-determining TS, the rate of insertion increases as the ancillary ligand becomes more electron rich or less sterically bulky. There is, however, no rate enhancement from Lewis acids (LA). In comparison, we establish that for an outersphere CO2 insertion, proposed to proceed with no interaction between CO2 and the metal in the rate-determining TS, there is a dramatic LA effect. Furthermore, for both inner- and outersphere reactions, we show that there is a small solvent effect on the rate of CO2 insertion. Solvents that have higher acceptor numbers generally lead to faster CO2 insertion. Our results provide an experimental method to determine the pathway for CO2 insertion and offer guidance for rate enhancement in CO2 reduction catalysis.
中文翻译:

加速 CO2 插入金属氢化物:配体、路易斯酸和溶剂对反应动力学的影响†
CO 2插入金属氢化物和金属甲酸盐的微观反向脱羧是CO 2加氢生成甲酸和甲醇以及甲酸和甲醇脱氢的催化循环中的重要基本步骤。在这里,我们使用快速混合停流技术来研究CO 2嵌入过渡金属氢化物的动力学和机制。研究发现,加速CO 2插入金属氢化物速率的最有效方法可能取决于决定速率的过渡态(TS) 的性质。我们证明,对于内球CO 2插入反应(其被认为在决定速率的TS中CO 2和金属之间存在直接相互作用),随着辅助配体变得更富电子或空间体积更小,插入速率增加。然而,路易斯酸 (LA) 不会提高速率。相比之下,我们确定,对于外球 CO 2插入,建议在决定速率的 TS 中CO 2和金属之间没有相互作用的情况下进行,存在显着的 LA 效应。此外,对于内层和外层反应,我们表明溶剂对CO 2插入速率的影响很小。具有较高受体数量的溶剂通常导致更快的CO 2插入。我们的结果提供了一种实验方法来确定CO 2插入途径,并为CO 2还原催化的速率提高提供指导。
更新日期:2018-07-06
中文翻译:

加速 CO2 插入金属氢化物:配体、路易斯酸和溶剂对反应动力学的影响†
CO 2插入金属氢化物和金属甲酸盐的微观反向脱羧是CO 2加氢生成甲酸和甲醇以及甲酸和甲醇脱氢的催化循环中的重要基本步骤。在这里,我们使用快速混合停流技术来研究CO 2嵌入过渡金属氢化物的动力学和机制。研究发现,加速CO 2插入金属氢化物速率的最有效方法可能取决于决定速率的过渡态(TS) 的性质。我们证明,对于内球CO 2插入反应(其被认为在决定速率的TS中CO 2和金属之间存在直接相互作用),随着辅助配体变得更富电子或空间体积更小,插入速率增加。然而,路易斯酸 (LA) 不会提高速率。相比之下,我们确定,对于外球 CO 2插入,建议在决定速率的 TS 中CO 2和金属之间没有相互作用的情况下进行,存在显着的 LA 效应。此外,对于内层和外层反应,我们表明溶剂对CO 2插入速率的影响很小。具有较高受体数量的溶剂通常导致更快的CO 2插入。我们的结果提供了一种实验方法来确定CO 2插入途径,并为CO 2还原催化的速率提高提供指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号