Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism for remodelling of the cell cycle checkpoint protein MAD2 by the ATPase TRIP13
Nature ( IF 64.8 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0281-1 Claudio Alfieri 1 , Leifu Chang 1, 2 , David Barford 1
Nature ( IF 64.8 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0281-1 Claudio Alfieri 1 , Leifu Chang 1, 2 , David Barford 1
Affiliation
|
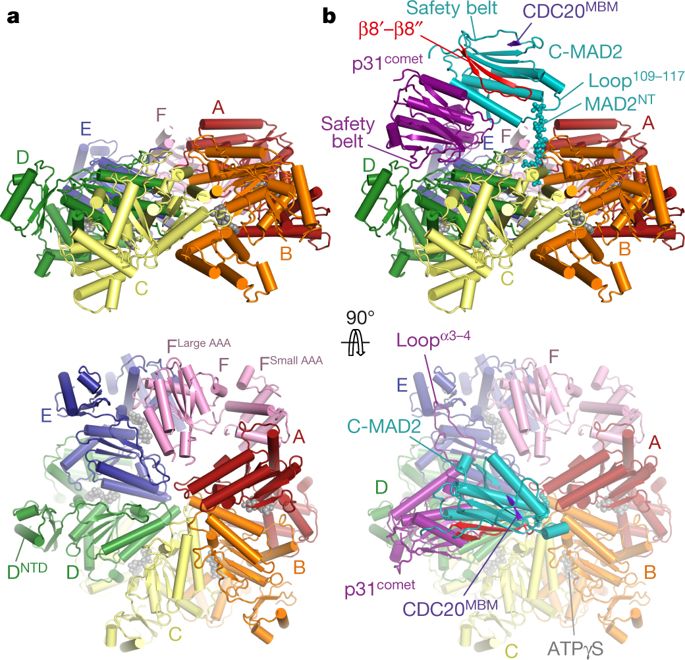
The maintenance of genome stability during mitosis is coordinated by the spindle assembly checkpoint (SAC) through its effector the mitotic checkpoint complex (MCC), an inhibitor of the anaphase-promoting complex (APC/C, also known as the cyclosome)1,2. Unattached kinetochores control MCC assembly by catalysing a change in the topology of the β-sheet of MAD2 (an MCC subunit), thereby generating the active closed MAD2 (C-MAD2) conformer3–5. Disassembly of free MCC, which is required for SAC inactivation and chromosome segregation, is an ATP-dependent process driven by the AAA+ ATPase TRIP13. In combination with p31comet, an SAC antagonist6, TRIP13 remodels C-MAD2 into inactive open MAD2 (O-MAD2)7–10. Here, we present a mechanism that explains how TRIP13–p31comet disassembles the MCC. Cryo-electron microscopy structures of the TRIP13–p31comet–C-MAD2–CDC20 complex reveal that p31comet recruits C-MAD2 to a defined site on the TRIP13 hexameric ring, positioning the N terminus of C-MAD2 (MAD2NT) to insert into the axial pore of TRIP13 and distorting the TRIP13 ring to initiate remodelling. Molecular modelling suggests that by gripping MAD2NT within its axial pore, TRIP13 couples sequential ATP-driven translocation of its hexameric ring along MAD2NT to push upwards on, and simultaneously rotate, the globular domains of the p31comet–C-MAD2 complex. This unwinds a region of the αA helix of C-MAD2 that is required to stabilize the C-MAD2 β-sheet, thus destabilizing C-MAD2 in favour of O-MAD2 and dissociating MAD2 from p31comet. Our study provides insights into how specific substrates are recruited to AAA+ ATPases through adaptor proteins and suggests a model of how translocation through the axial pore of AAA+ ATPases is coupled to protein remodelling.Structural analysis demonstrates how TRIP13 and p31comet disassemble the mitotic checkpoint complex.
中文翻译:

ATPase TRIP13重塑细胞周期检查点蛋白MAD2的机制
有丝分裂期间基因组稳定性的维持由纺锤体装配检查点 (SAC) 通过其效应器有丝分裂检查点复合物 (MCC) 进行协调,MCC 是后期促进复合物 (APC/C,也称为环体) 的抑制剂 1,2 . 未连接的着丝粒通过催化 MAD2(MCC 亚基)的 β-折叠拓扑结构的变化来控制 MCC 组装,从而产生活性闭合 MAD2(C-MAD2)构象异构体 3-5。SAC 失活和染色体分离所需的游离 MCC 的分解是由 AAA+ ATPase TRIP13 驱动的 ATP 依赖性过程。与 SAC 拮抗剂 p31comet 结合使用,TRIP13 将 C-MAD2 重塑为无活性的开放式 MAD2 (O-MAD2)7-10。在这里,我们提出了一种机制来解释 TRIP13-p31comet 如何分解 MCC。TRIP13–p31comet–C-MAD2–CDC20 复合物的冷冻电子显微镜结构显示 p31comet 将 C-MAD2 招募到 TRIP13 六聚体环上的特定位点,将 C-MAD2 (MAD2NT) 的 N 末端定位到插入轴向TRIP13 的孔并扭曲 TRIP13 环以启动重塑。分子模型表明,通过将 MAD2NT 夹在其轴向孔内,TRIP13 将其六聚体环沿 MAD2NT 的顺序 ATP 驱动易位耦合,以向上推动并同时旋转 p31comet-C-MAD2 复合物的球状结构域。这会展开 C-MAD2 的 αA 螺旋区域,该区域是稳定 C-MAD2 β-折叠所需的,从而使 C-MAD2 不稳定,有利于 O-MAD2,并使 MAD2 从 p31comet 中解离。
更新日期:2018-07-01
中文翻译:

ATPase TRIP13重塑细胞周期检查点蛋白MAD2的机制
有丝分裂期间基因组稳定性的维持由纺锤体装配检查点 (SAC) 通过其效应器有丝分裂检查点复合物 (MCC) 进行协调,MCC 是后期促进复合物 (APC/C,也称为环体) 的抑制剂 1,2 . 未连接的着丝粒通过催化 MAD2(MCC 亚基)的 β-折叠拓扑结构的变化来控制 MCC 组装,从而产生活性闭合 MAD2(C-MAD2)构象异构体 3-5。SAC 失活和染色体分离所需的游离 MCC 的分解是由 AAA+ ATPase TRIP13 驱动的 ATP 依赖性过程。与 SAC 拮抗剂 p31comet 结合使用,TRIP13 将 C-MAD2 重塑为无活性的开放式 MAD2 (O-MAD2)7-10。在这里,我们提出了一种机制来解释 TRIP13-p31comet 如何分解 MCC。TRIP13–p31comet–C-MAD2–CDC20 复合物的冷冻电子显微镜结构显示 p31comet 将 C-MAD2 招募到 TRIP13 六聚体环上的特定位点,将 C-MAD2 (MAD2NT) 的 N 末端定位到插入轴向TRIP13 的孔并扭曲 TRIP13 环以启动重塑。分子模型表明,通过将 MAD2NT 夹在其轴向孔内,TRIP13 将其六聚体环沿 MAD2NT 的顺序 ATP 驱动易位耦合,以向上推动并同时旋转 p31comet-C-MAD2 复合物的球状结构域。这会展开 C-MAD2 的 αA 螺旋区域,该区域是稳定 C-MAD2 β-折叠所需的,从而使 C-MAD2 不稳定,有利于 O-MAD2,并使 MAD2 从 p31comet 中解离。

























 京公网安备 11010802027423号
京公网安备 11010802027423号