当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel(ii)-promoted specific hydrolysis of zinc finger proteins†
Metallomics ( IF 3.4 ) Pub Date : 2018-07-04 00:00:00 , DOI: 10.1039/c8mt00098k Agnieszka Belczyk-Ciesielska 1, 2, 3, 4 , Brigitta Csipak 5, 6, 7, 8 , Bálint Hajdu 5, 6, 7, 8 , Aleksandra Sparavier 1, 2, 3, 4 , Masamitsu N. Asaka 9, 10, 11, 12, 13 , Kyosuke Nagata 9, 10, 11, 12, 13 , Béla Gyurcsik 5, 6, 7, 8 , Wojciech Bal 1, 2, 3, 4
Metallomics ( IF 3.4 ) Pub Date : 2018-07-04 00:00:00 , DOI: 10.1039/c8mt00098k Agnieszka Belczyk-Ciesielska 1, 2, 3, 4 , Brigitta Csipak 5, 6, 7, 8 , Bálint Hajdu 5, 6, 7, 8 , Aleksandra Sparavier 1, 2, 3, 4 , Masamitsu N. Asaka 9, 10, 11, 12, 13 , Kyosuke Nagata 9, 10, 11, 12, 13 , Béla Gyurcsik 5, 6, 7, 8 , Wojciech Bal 1, 2, 3, 4
Affiliation
|
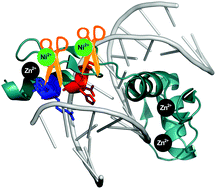
In this work we demonstrate that the previously described reaction of sequence specific Ni(II)-dependent hydrolytic peptide bond cleavage can be performed in complex metalloprotein molecules, such as the Cys2His2 zinc finger proteins. The cleavage within a zinc finger unit possessing a (Ser/Thr)-X-His sequence is not hindered by the presence of the Zn(II) ions. It results in loss of the Zn(II) ion, oxidation of the SH groups and thus, in a collapse of the functional structure. We show that such natural Ni(II)-cleavage sites in zinc finger domains can be edited out without compromising the DNA binding specificity. Inserting a Ni(II)-susceptible sequence between the edited zinc finger and an affinity tag allows for removal of the latter sequence by Ni(II) ions after the protein purification. We have shown that this reaction can be executed even when a metal ion binding N-terminal His-tag is present. The cleavage product maintains the native zinc finger structure involving Zn(II) ions. Mass spectra revealed that a Ni(II) ion remains coordinated to the hydrolyzed protein product through the N-terminal (Ser/Thr)-X-His tripeptide segment. The fact that the Ni(II)-dependent protein hydrolysis is influenced by the Ni(II) concentration, pH and temperature of the reaction provides a platform for novel regulated DNA effector design.
中文翻译:

镍(ii)促进锌指蛋白的特异性水解†
在这项工作中,我们证明了先前描述的序列特异性Ni(II)依赖性水解肽键裂解反应可在复杂的金属蛋白分子(例如Cys 2 His 2锌指蛋白)中进行。具有(Ser / Thr)-X-His序列的锌指单元内的裂解不受Zn(II)离子的存在的阻碍。其导致Zn(II)离子的损失,SH基团的氧化并因此导致功能结构的破坏。我们表明,锌指结构域中的这种天然的Ni(II)裂解位点可以被编辑掉而不会损害DNA结合特异性。插入Ni(II编辑的锌指和亲和标签之间的α-敏感序列允许在蛋白质纯化后通过Ni(II)离子去除后者的序列。我们已经表明,即使存在结合金属离子的N端His-tag,也可以进行该反应。裂解产物维持涉及Zn(II)离子的天然锌指结构。质谱显示,Ni(II)离子通过N端(Ser / Thr)-X-His三肽片段与水解蛋白产物保持配位。Ni(II)依赖性蛋白水解受反应的Ni(II)浓度,pH和温度影响的事实为新型调节DNA效应子设计提供了平台。
更新日期:2018-07-04
中文翻译:

镍(ii)促进锌指蛋白的特异性水解†
在这项工作中,我们证明了先前描述的序列特异性Ni(II)依赖性水解肽键裂解反应可在复杂的金属蛋白分子(例如Cys 2 His 2锌指蛋白)中进行。具有(Ser / Thr)-X-His序列的锌指单元内的裂解不受Zn(II)离子的存在的阻碍。其导致Zn(II)离子的损失,SH基团的氧化并因此导致功能结构的破坏。我们表明,锌指结构域中的这种天然的Ni(II)裂解位点可以被编辑掉而不会损害DNA结合特异性。插入Ni(II编辑的锌指和亲和标签之间的α-敏感序列允许在蛋白质纯化后通过Ni(II)离子去除后者的序列。我们已经表明,即使存在结合金属离子的N端His-tag,也可以进行该反应。裂解产物维持涉及Zn(II)离子的天然锌指结构。质谱显示,Ni(II)离子通过N端(Ser / Thr)-X-His三肽片段与水解蛋白产物保持配位。Ni(II)依赖性蛋白水解受反应的Ni(II)浓度,pH和温度影响的事实为新型调节DNA效应子设计提供了平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号