当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural and Computational Bases for Dramatic Skeletal Rearrangement in Anditomin Biosynthesis
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-04 , DOI: 10.1021/jacs.8b06084 Yu Nakashima 1 , Takaaki Mitsuhashi 1 , Yudai Matsuda 1, 2 , Miki Senda 3 , Hajime Sato 4, 5 , Mami Yamazaki 4 , Masanobu Uchiyama 1, 5 , Toshiya Senda 3, 6 , Ikuro Abe 1, 7
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-04 , DOI: 10.1021/jacs.8b06084 Yu Nakashima 1 , Takaaki Mitsuhashi 1 , Yudai Matsuda 1, 2 , Miki Senda 3 , Hajime Sato 4, 5 , Mami Yamazaki 4 , Masanobu Uchiyama 1, 5 , Toshiya Senda 3, 6 , Ikuro Abe 1, 7
Affiliation
|
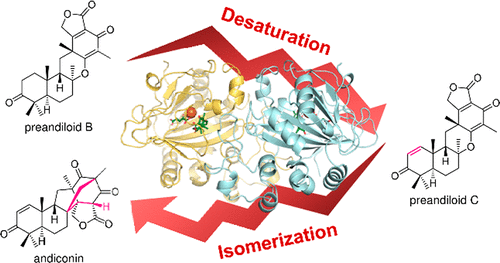
AndA, an Fe(II)/α-ketoglutarate (αKG)-dependent enzyme, is the key enzyme that constructs the unique and congested bridged-ring system of anditomin (1), by catalyzing consecutive dehydrogenation and isomerization reactions. Although we previously characterized AndA to some extent, the means by which the enzyme facilitates this drastic structural reconstruction have remained elusive. In this study, we have solved three X-ray crystal structures of AndA, in its apo form and in the complexes with Fe(II), αKG, and two substrates. The crystal structures and mutational experiments identified several key amino acid residues important for the catalysis and provided insight into how AndA controls the reaction. Furthermore, computational calculations validated the proposed reaction mechanism for the bridged-ring formation and also revealed the requirement of a series of conformational changes during the transformation.
中文翻译:

Anditomin 生物合成中显着骨架重排的结构和计算基础
AndA 是一种 Fe(II)/α-酮戊二酸 (αKG) 依赖性酶,是通过催化连续脱氢和异构化反应构建 anditomin (1) 独特而拥挤的桥环系统的关键酶。尽管我们之前在某种程度上表征了 AndA,但酶促进这种剧烈结构重建的方式仍然难以捉摸。在这项研究中,我们解决了 AndA 的三种 X 射线晶体结构,其 apo 形式以及与 Fe(II)、αKG 和两种底物的复合物。晶体结构和突变实验确定了几个对催化很重要的关键氨基酸残基,并提供了对 AndA 如何控制反应的见解。此外,
更新日期:2018-07-04
中文翻译:

Anditomin 生物合成中显着骨架重排的结构和计算基础
AndA 是一种 Fe(II)/α-酮戊二酸 (αKG) 依赖性酶,是通过催化连续脱氢和异构化反应构建 anditomin (1) 独特而拥挤的桥环系统的关键酶。尽管我们之前在某种程度上表征了 AndA,但酶促进这种剧烈结构重建的方式仍然难以捉摸。在这项研究中,我们解决了 AndA 的三种 X 射线晶体结构,其 apo 形式以及与 Fe(II)、αKG 和两种底物的复合物。晶体结构和突变实验确定了几个对催化很重要的关键氨基酸残基,并提供了对 AndA 如何控制反应的见解。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号