当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct kinetics study of CH2OO + methyl vinyl ketone and CH2OO + methacrolein reactions and an upper limit determination for CH2OO + CO reaction†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8cp03606c Arkke J. Eskola 1, 2, 3, 4, 5 , Malte Döntgen 4, 5, 6, 7 , Brandon Rotavera 1, 2, 3 , Rebecca L. Caravan 1, 2, 3 , Oliver Welz 1, 2, 3 , John D. Savee 1, 2, 3 , David L. Osborn 1, 2, 3 , Dudley E. Shallcross 8, 9, 10, 11 , Carl J. Percival 11, 12, 13, 14, 15 , Craig A. Taatjes 1, 2, 3
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8cp03606c Arkke J. Eskola 1, 2, 3, 4, 5 , Malte Döntgen 4, 5, 6, 7 , Brandon Rotavera 1, 2, 3 , Rebecca L. Caravan 1, 2, 3 , Oliver Welz 1, 2, 3 , John D. Savee 1, 2, 3 , David L. Osborn 1, 2, 3 , Dudley E. Shallcross 8, 9, 10, 11 , Carl J. Percival 11, 12, 13, 14, 15 , Craig A. Taatjes 1, 2, 3
Affiliation
|
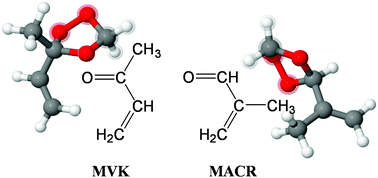
Methyl vinyl ketone (MVK) and methacrolein (MACR) are important intermediate products in atmospheric degradation of volatile organic compounds, especially of isoprene. This work investigates the reactions of the smallest Criegee intermediate, CH2OO, with its co-products from isoprene ozonolysis, MVK and MACR, using multiplexed photoionization mass spectrometry (MPIMS), with either tunable synchrotron radiation from the Advanced Light Source or Lyman-α (10.2 eV) radiation for photoionization. CH2OO was produced via pulsed laser photolysis of CH2I2 in the presence of excess O2. Time-resolved measurements of reactant disappearance and of product formation were performed to monitor reaction progress; first order rate coefficients were obtained from exponential fits to the CH2OO decays. The bimolecular reaction rate coefficients at 300 K and 4 Torr are k(CH2OO + MVK) = (5.0 ± 0.4) × 10−13 cm3 s−1 and k(CH2OO + MACR) = (4.4 ± 1.0) × 10−13 cm3 s−1, where the stated ±2σ uncertainties are statistical uncertainties. Adduct formation is observed for both reactions and is attributed to the formation of a secondary ozonides (1,2,4-trioxolanes), supported by master equation calculations of the kinetics and the agreement between measured and calculated adiabatic ionization energies. Kinetics measurements were also performed for a possible bimolecular CH2OO + CO reaction and for the reaction of CH2OO with CF3CHCH2 at 300 K and 4 Torr. For CH2OO + CO, no reaction is observed and an upper limit is determined: k(CH2OO + CO) < 2 × 10−16 cm3 s−1. For CH2OO + CF3CHCH2, an upper limit of k(CH2OO + CF3CHCH2) < 2 × 10−14 cm3 s−1 is obtained.
中文翻译:

CH 2 OO +甲基乙烯基酮和CH 2 OO +甲基丙烯醛反应的直接动力学研究以及CH 2 OO + CO反应的上限测定†
甲基乙烯基酮(MVK)和甲基丙烯醛(MACR)是挥发性有机化合物(尤其是异戊二烯)在大气中降解的重要中间产物。这项工作使用多重光电离质谱法(MPIMS),结合来自先进光源或莱曼光谱仪的可调同步辐射,研究了最小的Criegee中间体CH 2 OO与异戊二烯臭氧分解,MVK和MACR的副产物的反应。 α(10.2 eV)辐射用于光电离。在过量O 2的存在下通过CH 2 I 2的脉冲激光光解产生CH 2 OO。进行时间分辨的反应物消失和产物形成的测量,以监测反应进程。一阶速率系数是从对CH 2 OO衰减的指数拟合中获得的。在300 K和4 Torr下的双分子反应速率系数为k(CH 2 OO + MVK)=(5.0±0.4)×10 -13 cm 3 s -1和k(CH 2 OO + MACR)=(4.4±1.0) ×10 -13 cm 3 s -1,其中规定的± 2σ不确定性是统计不确定性。在两个反应中均观察到加合物的形成,这归因于形成了次级恶臭化物(1,2,4-三氧戊环),并由动力学的主方程计算以及绝热电离能之间的一致性所支持。还针对可能的双分子CH 2 OO + CO反应以及CH 2 OO与CF 3 CHCH 2在300 K和4 Torr下的反应进行了动力学测量。对于CH 2 OO + CO,未观察到反应,并且确定了上限:k(CH 2 OO + CO)<2×10 -16 cm 3 s -1。对于CH通过2 OO + CF 3 CHCH 2,得到k的上限(CH 2 OO + CF 3 CHCH 2)<2×10 -14 cm 3 s -1。
更新日期:2018-07-03
中文翻译:

CH 2 OO +甲基乙烯基酮和CH 2 OO +甲基丙烯醛反应的直接动力学研究以及CH 2 OO + CO反应的上限测定†
甲基乙烯基酮(MVK)和甲基丙烯醛(MACR)是挥发性有机化合物(尤其是异戊二烯)在大气中降解的重要中间产物。这项工作使用多重光电离质谱法(MPIMS),结合来自先进光源或莱曼光谱仪的可调同步辐射,研究了最小的Criegee中间体CH 2 OO与异戊二烯臭氧分解,MVK和MACR的副产物的反应。 α(10.2 eV)辐射用于光电离。在过量O 2的存在下通过CH 2 I 2的脉冲激光光解产生CH 2 OO。进行时间分辨的反应物消失和产物形成的测量,以监测反应进程。一阶速率系数是从对CH 2 OO衰减的指数拟合中获得的。在300 K和4 Torr下的双分子反应速率系数为k(CH 2 OO + MVK)=(5.0±0.4)×10 -13 cm 3 s -1和k(CH 2 OO + MACR)=(4.4±1.0) ×10 -13 cm 3 s -1,其中规定的± 2σ不确定性是统计不确定性。在两个反应中均观察到加合物的形成,这归因于形成了次级恶臭化物(1,2,4-三氧戊环),并由动力学的主方程计算以及绝热电离能之间的一致性所支持。还针对可能的双分子CH 2 OO + CO反应以及CH 2 OO与CF 3 CHCH 2在300 K和4 Torr下的反应进行了动力学测量。对于CH 2 OO + CO,未观察到反应,并且确定了上限:k(CH 2 OO + CO)<2×10 -16 cm 3 s -1。对于CH通过2 OO + CF 3 CHCH 2,得到k的上限(CH 2 OO + CF 3 CHCH 2)<2×10 -14 cm 3 s -1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号