当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
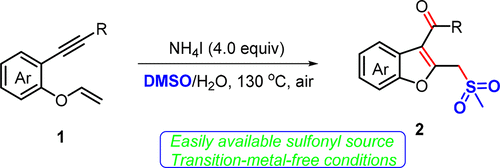
Radical Addition Cascade Cyclization of 1,6-Enynes with DMSO To Access Methylsulfonylated and Carbonylated Benzofurans under Transition-Metal-Free Conditions
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.joc.8b01265 Jie Zhang 1 , Shijing Cheng 1 , Zhiqiang Cai 1 , Ping Liu 1 , Peipei Sun 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.joc.8b01265 Jie Zhang 1 , Shijing Cheng 1 , Zhiqiang Cai 1 , Ping Liu 1 , Peipei Sun 1
Affiliation
|
A mild and direct addition/cyclization cascade to construct methylsulfonylated and carbonylated benzofurans was accomplished using oxygen-linked 1,6-enynes as the starting materials. The interaction of NH4I and DMSO promoted the generation of sulfur-containing radical and initiated the radical addition to the C═C double bond of 1,6-enyne cascade intramolecular 5-exo-dig cyclization. A wide range of oxygen-linked 1,6-enynes bearing various substituents were found to be suitable in this cascade process, providing benzofurans with dual functional groups in moderate to high yields. This method could also be utilized to synthesize benzothiophenes from sulfur-linked 1,6-enynes.
中文翻译:

在无过渡金属的条件下,用DMSO进行1,6-炔烃的自由基加成级联反应,以得到甲基磺酰化和碳酰化的苯并呋喃
使用氧连接的1,6-炔烃作为起始原料,完成了温和而直接的加成/环化级联反应,以构建甲基磺酰化和羰基化的苯并呋喃。NH的相互作用4 I和DMSO促进的产生自由基和引发自由基加成到的1,6-烯炔级联的分子内5- C = C双键的含硫外型-挖环化。已发现,带有各种取代基的多种与氧连接的1,6-烯炔适合用于该级联过程,以中等至高收率提供具有双官能团的苯并呋喃。该方法也可用于由硫连接的1,6-烯炔合成苯并噻吩。
更新日期:2018-07-03
中文翻译:

在无过渡金属的条件下,用DMSO进行1,6-炔烃的自由基加成级联反应,以得到甲基磺酰化和碳酰化的苯并呋喃
使用氧连接的1,6-炔烃作为起始原料,完成了温和而直接的加成/环化级联反应,以构建甲基磺酰化和羰基化的苯并呋喃。NH的相互作用4 I和DMSO促进的产生自由基和引发自由基加成到的1,6-烯炔级联的分子内5- C = C双键的含硫外型-挖环化。已发现,带有各种取代基的多种与氧连接的1,6-烯炔适合用于该级联过程,以中等至高收率提供具有双官能团的苯并呋喃。该方法也可用于由硫连接的1,6-烯炔合成苯并噻吩。



























 京公网安备 11010802027423号
京公网安备 11010802027423号