当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How a Solvent Molecule Affects Competing Elimination and Substitution Dynamics. Insight into Mechanism Evolution with Increased Solvation
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-03 , DOI: 10.1021/jacs.8b04529 Xu Liu 1 , Jiaxu Zhang 1 , Li Yang 1 , William L. Hase 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-07-03 , DOI: 10.1021/jacs.8b04529 Xu Liu 1 , Jiaxu Zhang 1 , Li Yang 1 , William L. Hase 2
Affiliation
|
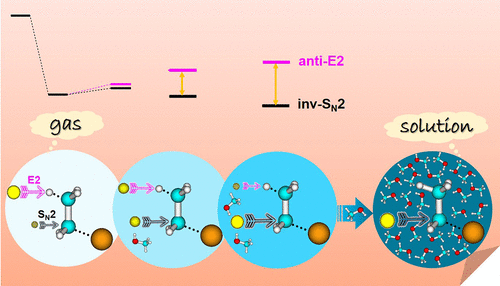
Competiting SN2 substitution and E2 elimination reactions are of central importance in preparative organic synthesis. Here, we unravel how individual solvent molecules may affect underlying SN2/E2 atomistic dynamics, which remains largely unclear with respective to their effects on reactivity. Results are presented for a prototype microsolvated case of fluoride anion reacting with ethyl bromide. Reaction dynamics simulations reproduce experimental findings at near thermal energies and show that the E2 mechanism dominates over SN2 for solvent-free reaction. This is energetically quite unexpected and results from dynamical effects. Adding one solvating methanol molecule introduces strikingly distinct dynamical behaviors that largely promote the SN2 reaction, a feature which attributes to a differential solute-solvent interaction at the central barrier that more strongly stabilizes the transition state for substitution. Upon further solvation, this enhanced stabilization of the SN2 mechanism becomes more pronounced, concomitant with drastic suppression of the E2 route. This work highlights the interplay between energetics and dynamics in determining mechanistic selectivity and provides insight into the impact of solvent molecules on a general transition from elimination to substitution for chemical reactions proceeding from gas- to solution-phase environments.
中文翻译:

溶剂分子如何影响竞争消除和替代动力学。深入了解溶解度增加的机理演变
竞争性 SN2 取代和 E2 消除反应在制备性有机合成中至关重要。在这里,我们解开了单个溶剂分子如何影响潜在的 SN2/E2 原子动力学,这在很大程度上仍不清楚它们对反应性的影响。结果显示为氟阴离子与乙基溴反应的原型微溶剂化情况。反应动力学模拟再现了接近热能的实验结果,并表明 E2 机制在无溶剂反应中优于 SN2。这在能量上是出乎意料的,并且是由动力效应引起的。添加一个溶剂化甲醇分子会引入显着不同的动力学行为,在很大程度上促进了 SN2 反应,归因于中心势垒处不同的溶质 - 溶剂相互作用的特征,可以更强烈地稳定替代过渡态。在进一步溶解后,SN2 机制的这种增强的稳定性变得更加明显,伴随着对 E2 路线的剧烈抑制。这项工作突出了能量学和动力学在确定机械选择性方面的相互作用,并提供了对溶剂分子对从气相环境到液相环境进行的化学反应从消除到替代的一般转变的影响的见解。
更新日期:2018-07-03
中文翻译:

溶剂分子如何影响竞争消除和替代动力学。深入了解溶解度增加的机理演变
竞争性 SN2 取代和 E2 消除反应在制备性有机合成中至关重要。在这里,我们解开了单个溶剂分子如何影响潜在的 SN2/E2 原子动力学,这在很大程度上仍不清楚它们对反应性的影响。结果显示为氟阴离子与乙基溴反应的原型微溶剂化情况。反应动力学模拟再现了接近热能的实验结果,并表明 E2 机制在无溶剂反应中优于 SN2。这在能量上是出乎意料的,并且是由动力效应引起的。添加一个溶剂化甲醇分子会引入显着不同的动力学行为,在很大程度上促进了 SN2 反应,归因于中心势垒处不同的溶质 - 溶剂相互作用的特征,可以更强烈地稳定替代过渡态。在进一步溶解后,SN2 机制的这种增强的稳定性变得更加明显,伴随着对 E2 路线的剧烈抑制。这项工作突出了能量学和动力学在确定机械选择性方面的相互作用,并提供了对溶剂分子对从气相环境到液相环境进行的化学反应从消除到替代的一般转变的影响的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号