当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Strategic C to N Replacement in β-Peptides: Atomic Level Control of Helical Folding
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.joc.8b01096 Valérie Declerck 1 , David J. Aitken 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.joc.8b01096 Valérie Declerck 1 , David J. Aitken 1
Affiliation
|
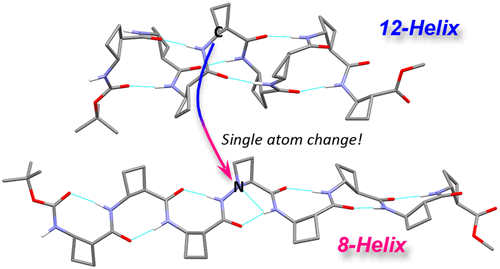
Single residue control of the helical topology of β-peptides is a contemporary challenge in foldamer science. We present the conformational preferences of oligomers of trans-2-aminocyclobutanecarboxylic acid (tACBC), in which a central residue has been replaced by a single N-aminoazetidine-2-carboxylic acid (AAzC) moiety. The latter has such a strong demand for local 8-helical conformers that the usual 12-helix secondary structure of a tACBC octamer is switched to a fully 8-helical conformation as a result of the single residue substitution.
中文翻译:

β肽的战略性C到N替换:螺旋折叠的原子水平控制。
对β-肽的螺旋形拓扑的单残基控制是折叠科学中的当代挑战。我们提出反式-2-氨基环丁烷羧酸(t ACBC)的低聚物的构象偏好,其中中心残基已被单个N-氨基氮杂环丁烷-2-羧酸(AAzC)部分取代。后者对局部8-螺旋构象异构体有如此强烈的需求,以至于t ACBC八聚体的通常的12-螺旋二级结构由于单个残基取代而转变为完全8-螺旋构象。
更新日期:2018-07-02
中文翻译:

β肽的战略性C到N替换:螺旋折叠的原子水平控制。
对β-肽的螺旋形拓扑的单残基控制是折叠科学中的当代挑战。我们提出反式-2-氨基环丁烷羧酸(t ACBC)的低聚物的构象偏好,其中中心残基已被单个N-氨基氮杂环丁烷-2-羧酸(AAzC)部分取代。后者对局部8-螺旋构象异构体有如此强烈的需求,以至于t ACBC八聚体的通常的12-螺旋二级结构由于单个残基取代而转变为完全8-螺旋构象。


























 京公网安备 11010802027423号
京公网安备 11010802027423号