当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of an Efficient Manufacturing Process for Reversible Bruton’s Tyrosine Kinase Inhibitor GDC-0853
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.oprd.8b00134 Haiming Zhang 1 , Theresa Cravillion 1 , Ngiap-Kie Lim 1 , Qingping Tian 1 , Danial Beaudry 1 , Jessica L. Defreese 1 , Alec Fettes , Philippe James , David Linder , Sushant Malhotra 1 , Chong Han 1 , Remy Angelaud 1 , Francis Gosselin 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.oprd.8b00134 Haiming Zhang 1 , Theresa Cravillion 1 , Ngiap-Kie Lim 1 , Qingping Tian 1 , Danial Beaudry 1 , Jessica L. Defreese 1 , Alec Fettes , Philippe James , David Linder , Sushant Malhotra 1 , Chong Han 1 , Remy Angelaud 1 , Francis Gosselin 1
Affiliation
|
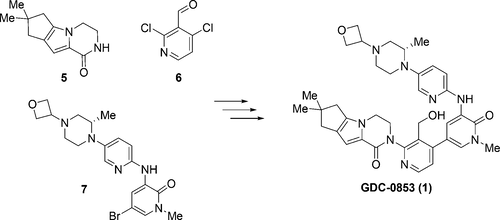
Efforts toward the process development of reversible Bruton’s tyrosine kinase (BTK) inhibitor GDC-0853 (1) are described. A practical synthesis of GDC-0853 was accomplished via a key highly regioselective Pd-catalyzed C–N coupling of tricyclic lactam 5 with 2,4-dichloronicotinaldehyde (6) to afford the C–N coupling product 3, a Suzuki–Miyaura cross-coupling of intermediate 3 with boronic ester 4 derived from a Pd-catalyzed borylation of tetracyclic bromide 7, to generate penultimate aldehyde intermediate 2 and subsequent aldehyde reduction and recrystallization. Process development of starting materials 5, 6, and 7 is also discussed.
中文翻译:

开发可逆的布鲁顿酪氨酸激酶抑制剂GDC-0853的高效生产工艺
描述了对可逆布鲁顿酪氨酸激酶(BTK)抑制剂GDC-0853(1)的开发过程的努力。通过关键的高区域选择性Pd催化的三环内酰胺5与2,4-二氯烟碱醛(6)的C-N偶联,完成了GDC-0853的实际合成,从而得到了C-N偶联产物3,即Suzuki-Miyaura交联。将中间体3与衍生自四环溴化物7的Pd催化硼化的硼酸酯4偶联,生成倒数第二的醛中间体2,然后进行醛还原和重结晶。起始原料的工艺开发5,6和7也进行了讨论。
更新日期:2018-07-02
中文翻译:

开发可逆的布鲁顿酪氨酸激酶抑制剂GDC-0853的高效生产工艺
描述了对可逆布鲁顿酪氨酸激酶(BTK)抑制剂GDC-0853(1)的开发过程的努力。通过关键的高区域选择性Pd催化的三环内酰胺5与2,4-二氯烟碱醛(6)的C-N偶联,完成了GDC-0853的实际合成,从而得到了C-N偶联产物3,即Suzuki-Miyaura交联。将中间体3与衍生自四环溴化物7的Pd催化硼化的硼酸酯4偶联,生成倒数第二的醛中间体2,然后进行醛还原和重结晶。起始原料的工艺开发5,6和7也进行了讨论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号