当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
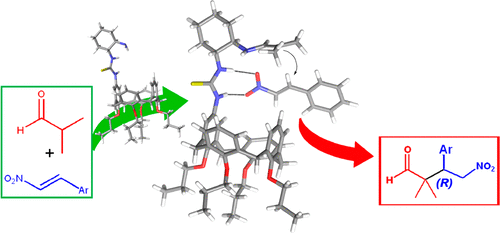
Solvent-Free Enantioselective Michael Reactions Catalyzed by a Calixarene-Based Primary Amine Thiourea
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-01 00:00:00 , DOI: 10.1021/acs.joc.8b01454 Nicola Alessandro De Simone 1 , Sara Meninno 1 , Carmen Talotta 1 , Carmine Gaeta 1 , Placido Neri 1 , Alessandra Lattanzi 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-01 00:00:00 , DOI: 10.1021/acs.joc.8b01454 Nicola Alessandro De Simone 1 , Sara Meninno 1 , Carmen Talotta 1 , Carmine Gaeta 1 , Placido Neri 1 , Alessandra Lattanzi 1
Affiliation
|
An upper-rim functionalized calix[4]arene-based thiourea installed onto the (R,R)-1,2-cyclohexanediamine scaffold was synthesized with a view to investigate its catalytic ability in enantioselective Michael additions. The reactions were found to conveniently proceed under solvent-free conditions, observing good to high enantioselectivities. From this preliminary study, the calix[4]arene unit is likely to play a role in affecting the conversion and to a lesser extent to the stereochemical outcome of the reactions through van der Waals contacts and C–H···π interactions with the substrates.
中文翻译:

基于杯芳烃的伯胺硫脲催化的无溶剂对映选择性迈克尔反应
为了研究其在对映选择性迈克尔加成反应中的催化能力,合成了安装在(R,R)-1,2-环己二胺支架上的上边缘功能化杯[4]芳烃基硫脲。发现反应在无溶剂条件下方便地进行,观察到良好至高对映选择性。根据这项初步研究,杯芳烃[4]芳烃单元可能会通过范德华接触和C–H···π相互作用与范德华接触并在较小程度上影响反应的立体化学结果,从而影响转化率。基材。
更新日期:2018-07-01
中文翻译:

基于杯芳烃的伯胺硫脲催化的无溶剂对映选择性迈克尔反应
为了研究其在对映选择性迈克尔加成反应中的催化能力,合成了安装在(R,R)-1,2-环己二胺支架上的上边缘功能化杯[4]芳烃基硫脲。发现反应在无溶剂条件下方便地进行,观察到良好至高对映选择性。根据这项初步研究,杯芳烃[4]芳烃单元可能会通过范德华接触和C–H···π相互作用与范德华接触并在较小程度上影响反应的立体化学结果,从而影响转化率。基材。



























 京公网安备 11010802027423号
京公网安备 11010802027423号