Electrochemistry Communications ( IF 5.4 ) Pub Date : 2018-07-02 , DOI: 10.1016/j.elecom.2018.06.014 Yih-Chyng Wu , Pierre-Louis Taberna , Patrice Simon
|
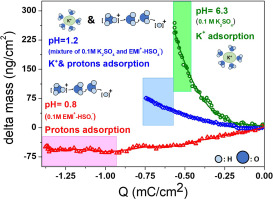
Electrochemical quartz crystal microbalance (EQCM) and cyclic voltammetry (CV) techniques were used to study ion dynamics in porous carbide-derived carbon (CDC) electrodes in various aqueous electrolytes. Although the cyclic voltammetries look similar, EQCM revealed different ion transfer depending on the electrolyte during both positive and negative polarization. During polarization in neutral K2SO4 electrolyte, partial desolvation of cation and anion were observed in carbon micropores. In EMI+-HSO4− electrolyte, the main charge carrier during cation adsorption was not found to be bulkier EMI+, but smaller and highly mobile H+. Furthermore, ionic fluxes during charging/discharging were monitored and identified in multi-ions aqueous system, which was ambiguous according to the CV plots. EQCM shows its powerful ability to serve as an accurate gravimetry probe to study the electrolyte concentration and compositional changes in porous materials.
中文翻译:

跟踪不同pH条件下的水性电解质混合物中多孔碳电极中的离子通量
电化学石英晶体微天平(EQCM)和循环伏安(CV)技术用于研究各种水性电解质中多孔碳化物衍生碳(CDC)电极中的离子动力学。尽管循环伏安看上去相似,但EQCM在正极化和负极化过程中均显示出取决于电解质的不同离子转移。在中性K 2 SO 4电解质中极化期间,在碳微孔中观察到阳离子和阴离子的部分去溶剂化。在EMI + -HSO 4 -电解质,阳离子吸附在主要载流子没有被发现是笨重EMI +,但更小且高度流动氢+。此外,在多离子水系统中对充电/放电过程中的离子通量进行了监测和识别,根据CV图,该通量是不明确的。EQCM表现出强大的功能,可以用作精确的重量分析探针来研究多孔材料中的电解质浓度和组成变化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号