当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biodegradable Synthetic Organelles Demonstrate ROS Shielding in Human-Complex-I-Deficient Fibroblasts
ACS Central Science ( IF 18.2 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acscentsci.8b00336 Lisanne M. P. E. van Oppen 1, 2 , Loai K. E. A. Abdelmohsen 3 , Sjenet E. van Emst-de Vries 1 , Pascal L. W. Welzen 3 , Daniela A. Wilson 4 , Jan A. M. Smeitink 2 , Werner J. H. Koopman 1 , Roland Brock 1 , Peter H. G. M. Willems 1 , David S. Williams 5 , Jan C.M. van Hest 3
ACS Central Science ( IF 18.2 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acscentsci.8b00336 Lisanne M. P. E. van Oppen 1, 2 , Loai K. E. A. Abdelmohsen 3 , Sjenet E. van Emst-de Vries 1 , Pascal L. W. Welzen 3 , Daniela A. Wilson 4 , Jan A. M. Smeitink 2 , Werner J. H. Koopman 1 , Roland Brock 1 , Peter H. G. M. Willems 1 , David S. Williams 5 , Jan C.M. van Hest 3
Affiliation
|
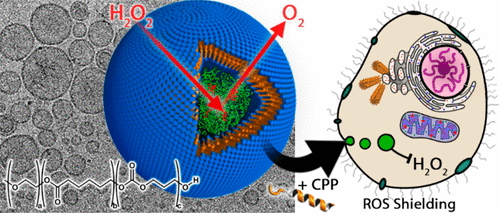
Biodegradable, semipermeable nanoreactors that are capable of undergoing cellular integration and, subsequently, function as synthetic organelles represent an exciting therapeutic technology. Polymersomal nanoreactors have been investigated as a suitable candidate for the engineering of such a system, with the chemical versatility and structural robustness required for such a demanding application. Although steps have been taken to demonstrate this capacity, there has yet to be a system presented with biochemically robust data showing therapeutic efficacy in primary human cells. The reason for this shortfall is the absence of essential criteria of the polymersomes tested so far; biodegradability, intrinsic semipermeability, and a biomedically relevant setting. Herein, we present enzyme-loaded, biodegradable poly(ethylene glycol)-block-poly(caprolactone-gradient-trimethylene carbonate) (PEG–PCLgTMC) polymersomal nanoreactors, readily fabricated using the biocompatible direct hydration methodology. Physical characterization of PEG–PCLgTMC polymersomes highlights their semipermeable membrane and ability to shield enzymatic cargo. Surface modification with cell-penetrating peptides (CPPs) directs cellular integration of enzyme-loaded PEG–PCLgTMC nanoreactors in a controlled fashion. Using HEK293T cells and human skin fibroblasts we demonstrate that biocompatible catalase nanoreactors successfully perform as a synthetic organelle, imparting activity-dependent antioxidant (reactive-oxygen-species-shielding, ROS-shielding) capacity to cells. Notably, for the first time, patient-derived human-complex-I-deficient primary fibroblasts are effectively protected against the toxicity of exogenous H2O2 by the action of internalized enzyme-loaded nanoreactors, showcasing this system in a therapeutically relevant context.
中文翻译:

可生物降解的合成细胞器显示人类复杂I缺陷成纤维细胞中的ROS屏蔽。
能够进行细胞整合并随后充当合成细胞器的可生物降解的半透性纳米反应器代表了一种令人兴奋的治疗技术。已经研究了聚合物体纳米反应器作为此类系统工程的合适候选者,具有此类苛刻应用所需的化学多功能性和结构坚固性。尽管已经采取步骤证明了这种能力,但是还没有一个系统显示出生化稳健的数据,该数据显示了对人类原代细胞的治疗功效。造成这种不足的原因是,到目前为止,缺乏测试的聚合物囊泡的基本标准。生物降解性,固有的半渗透性和生物医学相关的设置。在这里,我们介绍了酶负载的,可生物降解的聚乙二醇-嵌段-聚(己内酯-梯度碳酸亚丙酯)(PEG-PCL g TMC)聚合物型纳米反应器,可使用生物相容性直接水合方法轻松制备。PEG-PCL g TMC聚合体的物理特征突出了它们的半透膜和屏蔽酶货物的能力。用细胞穿透肽(CPP)进行表面修饰可指导酶负载的PEG–PCL g的细胞整合可控方式的TMC纳米反应器。使用HEK293T细胞和人类皮肤成纤维细胞,我们证明了生物相容性过氧化氢酶纳米反应器成功地发挥了合成细胞器的作用,赋予细胞依赖活性的抗氧化剂(活性氧物种屏蔽,ROS屏蔽)能力。值得注意的是,患者内源性人类复合物I缺陷型原代成纤维细胞首次通过内在化酶负载的纳米反应器的作用得到有效保护,免受外源H 2 O 2的毒性,从而在治疗上相关的背景下展示了该系统。
更新日期:2018-07-02
中文翻译:

可生物降解的合成细胞器显示人类复杂I缺陷成纤维细胞中的ROS屏蔽。
能够进行细胞整合并随后充当合成细胞器的可生物降解的半透性纳米反应器代表了一种令人兴奋的治疗技术。已经研究了聚合物体纳米反应器作为此类系统工程的合适候选者,具有此类苛刻应用所需的化学多功能性和结构坚固性。尽管已经采取步骤证明了这种能力,但是还没有一个系统显示出生化稳健的数据,该数据显示了对人类原代细胞的治疗功效。造成这种不足的原因是,到目前为止,缺乏测试的聚合物囊泡的基本标准。生物降解性,固有的半渗透性和生物医学相关的设置。在这里,我们介绍了酶负载的,可生物降解的聚乙二醇-嵌段-聚(己内酯-梯度碳酸亚丙酯)(PEG-PCL g TMC)聚合物型纳米反应器,可使用生物相容性直接水合方法轻松制备。PEG-PCL g TMC聚合体的物理特征突出了它们的半透膜和屏蔽酶货物的能力。用细胞穿透肽(CPP)进行表面修饰可指导酶负载的PEG–PCL g的细胞整合可控方式的TMC纳米反应器。使用HEK293T细胞和人类皮肤成纤维细胞,我们证明了生物相容性过氧化氢酶纳米反应器成功地发挥了合成细胞器的作用,赋予细胞依赖活性的抗氧化剂(活性氧物种屏蔽,ROS屏蔽)能力。值得注意的是,患者内源性人类复合物I缺陷型原代成纤维细胞首次通过内在化酶负载的纳米反应器的作用得到有效保护,免受外源H 2 O 2的毒性,从而在治疗上相关的背景下展示了该系统。


























 京公网安备 11010802027423号
京公网安备 11010802027423号