当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron‐Catalyzed Synthesis of the Hexahydrocyclopenta[c]furan Core and Concise Total Synthesis of Polyflavanostilbene B
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-07-16 , DOI: 10.1002/anie.201804329 Xujie Wang 1 , Fu Liu 1 , Juping Yun 1 , Ziming Feng 1 , Jianshuang Jiang 1 , Yanan Yang 1 , Peicheng Zhang 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-07-16 , DOI: 10.1002/anie.201804329 Xujie Wang 1 , Fu Liu 1 , Juping Yun 1 , Ziming Feng 1 , Jianshuang Jiang 1 , Yanan Yang 1 , Peicheng Zhang 1
Affiliation
|
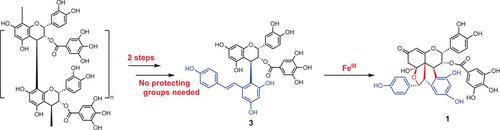
The first synthesis of polyflavanostilbene B (1), which has seven contiguous stereocenters including two quaternary carbon centers, from abundant polymeric (−)‐epicatechin gallate on a gram scale in three steps without the use of protecting groups is reported. The key transformations of this strategy include a regioselective and stereoselective substitution of resveratrol to give the 4‐derivative of (−)‐epicatechin 3‐gallate and an iron‐catalyzed cyclization reaction. The possible radical cyclization mechanism in the formation of the hexahydrocyclopenta[c]furan core is also discussed.
中文翻译:

铁催化六氢环戊五烯[c]呋喃核的合成及全黄酮B的简捷合成
据报道,在不使用保护基的情况下,可以在3步内由克数丰富的聚合(-)-表儿茶素没食子酸酯以3个步骤合成具有七个连续立体中心(包括两个季碳中心)的聚黄烷二茂烯B(1)。该策略的关键转变包括白藜芦醇的区域选择性和立体选择性取代,生成(-)-表儿茶素3-没食子酸酯的4-衍生物和铁催化的环化反应。还讨论了六氢环戊[c]呋喃核形成中可能的自由基环化机理。
更新日期:2018-07-16
中文翻译:

铁催化六氢环戊五烯[c]呋喃核的合成及全黄酮B的简捷合成
据报道,在不使用保护基的情况下,可以在3步内由克数丰富的聚合(-)-表儿茶素没食子酸酯以3个步骤合成具有七个连续立体中心(包括两个季碳中心)的聚黄烷二茂烯B(1)。该策略的关键转变包括白藜芦醇的区域选择性和立体选择性取代,生成(-)-表儿茶素3-没食子酸酯的4-衍生物和铁催化的环化反应。还讨论了六氢环戊[c]呋喃核形成中可能的自由基环化机理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号