Biomaterials ( IF 14.0 ) Pub Date : 2018-07-02 , DOI: 10.1016/j.biomaterials.2018.06.019 Akihiro Nishiguchi , Michiya Matsusaki , Mitsunobu R. Kano , Hiroshi Nishihara , Daisuke Okano , Yoshiya Asano , Hiroshi Shimoda , Satoko Kishimoto , Soichi Iwai , Mitsuru Akashi
|
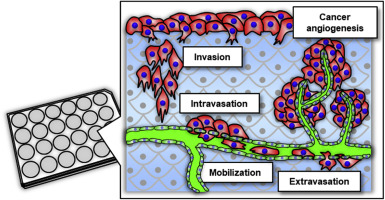
Tumour models mimicking in vivo three-dimensional (3D) microenvironments are of increasing interest in drug discovery because of the limitations inherent to current models. For example, preclinical assays that rely on monolayer or spheroid cell cultures cannot easily predict 3D cancer behaviours because they have no vasculature. Furthermore, there are major differences in cancer behaviour between human and animal experiments. Here, we show the construction of 3D blood/lymph-vascularized human stromal tissues that can be combined with cancer cells to mimic dynamic metastasis for real-time throughput screening of secreted proteinases. We validated this tool using three human carcinoma cell types that are known to invade blood/lymph vessels and promote angiogenesis. These cell types exhibited characteristic haematogenous/lymphogenous metastasis and tumour angiogenesis properties. Importantly, these carcinoma cells selectively secreted different matrix metalloproteinases depending on their metastasis stage and target vasculature, suggesting the possibility of developing drugs that can target each secreted proteinase. We conclude that the 3D tissue tool will be a powerful throughput system for predicting cancer cell responses and time-dependent secretion of molecules in preclinical assays.
中文翻译:

用于癌症转移的临床前测定的体外3D血液/淋巴血管化人体基质组织
模拟体内的肿瘤模型由于当前模型固有的局限性,三维(3D)微环境在药物开发中的兴趣日益浓厚。例如,依赖于单层或球状细胞培养物的临床前测定无法轻松预测3D癌症行为,因为它们没有脉管系统。此外,人与动物实验之间在癌症行为方面也存在重大差异。在这里,我们展示了3D血液/淋巴管形成的人类基质组织的构建,该组织可以与癌细胞结合以模拟动态转移,以实时检测分泌的蛋白酶。我们使用三种人类癌细胞类型验证了此工具,这些癌细胞类型可侵犯血液/淋巴管并促进血管生成。这些细胞类型表现出特征性的血源性/淋巴源性转移和肿瘤血管生成特性。重要的是,这些癌细胞根据其转移阶段和靶标脉管系统选择性分泌不同的基质金属蛋白酶,提示开发针对每种分泌的蛋白酶的药物的可能性。我们得出的结论是,3D组织工具将是一个功能强大的吞吐量系统,用于在临床前测定中预测癌细胞反应和分子的时间依赖性分泌。



























 京公网安备 11010802027423号
京公网安备 11010802027423号