当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multihierarchically Profiling the Biological Effects of Various Metal-Based Nanoparticles in Macrophages under Low Exposure Doses
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-06-30 00:00:00 , DOI: 10.1021/acssuschemeng.8b01744 Jie Zhang 1, 2 , Shunhao Wang 1, 2 , Ming Gao 1, 2 , Ruibin Li 3, 4 , Sijin Liu 1, 2
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-06-30 00:00:00 , DOI: 10.1021/acssuschemeng.8b01744 Jie Zhang 1, 2 , Shunhao Wang 1, 2 , Ming Gao 1, 2 , Ruibin Li 3, 4 , Sijin Liu 1, 2
Affiliation
|
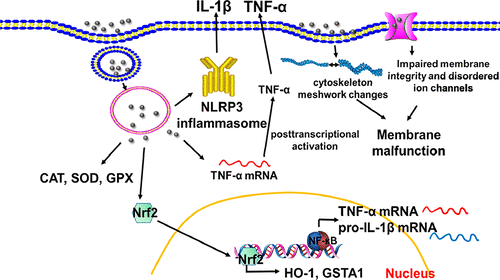
Thus far, tremendous efforts have been made to understand the biosafety of metal-based nanoparticles (MNPs). Nevertheless, most previous studies focused on specific adverse outcomes of MNPs at unrealistically high concentrations with little relevance to the National Institute for Occupational Safety and Health (NIOSH) exposure thresholds, and failed to comprehensively evaluate their toxicity profiles. To address these challenges, we here endeavored to multihierarchically profile the hazard effects of various popularly used MNPs in macrophages under low exposure doses. At these doses, no remarkable cell viability drop and cell death were induced. However, a cellular antioxidant defense system was seen to be initiated in cells by all MNPs even at these low concentrations, albeit to a differential extent and through different pathways, as reflected by differential induction of the antioxidant enzymes and Nrf2 signaling. Regarding inflammation, rare earth oxide nanomaterials (REOs) except nCeO2 greatly increased IL-1β secretion in a NLRP3 inflammasome-dependent manner. By contrast, six REOs, AgNP-5nm, nFe2O3, nFe3O4, and nZnO were found to elevate TNF-α concentration through post-transcriptional regulation. Moreover, all MNPs except nCeO2 drastically altered cellular membrane/cytoskeleton meshwork, but leading to different outcomes, with condensed cellular size and reduced numbers of protrusions by REOs and elongated protrusions by other MNPs. Consequently, REOs (e.g., nDy2O3 and nSm2O3) impaired phagocytosis of macrophages, and other MNPs (such as AgNP-25nm and nZnO) reversely enhanced macrophagic phagocytosis. Alterations of membrane and cytoskeleton meshwork induced by these MNPs also caused disordered membrane potential and calcium ion flux. Collectively, our data profiled the biological effects of different MNPs in macrophages under low exposure doses, and deciphered a complex network that links multiparallel pathways and processes to differential adverse outcomes.
中文翻译:

在低剂量下对巨噬细胞中各种基于金属的纳米粒子的生物效应进行多层次分析
迄今为止,已经做出了巨大的努力来理解金属基纳米颗粒(MNP)的生物安全性。尽管如此,以前的大多数研究都集中在浓度不切实际的高浓度MNPs的特定不良后果上,与国家职业安全与健康研究所(NIOSH)的暴露阈值几乎没有关系,并且未能全面评估其毒性特征。为了应对这些挑战,我们在这里努力在低暴露剂量下多层次地分析巨噬细胞中各种普遍使用的MNP的危害作用。在这些剂量下,没有引起明显的细胞活力下降和细胞死亡。但是,即使在低浓度下,所有MNP都可以在细胞中启动细胞抗氧化剂防御系统,尽管程度不同且途径不同,如抗氧化酶和Nrf2信号转导的差异诱导所反映的。关于炎症,除nCeO外,稀土氧化物纳米材料(REO)2以NLRP3炎性小体依赖性方式大大增加了IL-1β分泌。相比之下,发现六个REOs AgNP-5nm,nFe 2 O 3,nFe 3 O 4和nZnO通过转录后调节来提高TNF-α的浓度。此外,除nCeO 2以外的所有MNP都极大地改变了细胞膜/细胞骨架的网状结构,但导致了不同的结果,细胞大小缩小,REO突出的突起数量减少,其他MNP突出的突起数量减少。因此,REO(例如nDy 2 O 3和nSm 2 O 3)巨噬细胞的吞噬功能受损,而其他MNP(例如AgNP-25nm和nZnO)则反过来增强了巨噬细胞的吞噬能力。这些MNP诱导的膜和细胞骨架网状结构的改变也会引起膜电位和钙离子通量的紊乱。总的来说,我们的数据概述了低暴露剂量下巨噬细胞中不同MNP的生物学效应,并破译了一个复杂的网络,该网络将多重平行的途径和过程与不同的不良后果联系起来。
更新日期:2018-06-30
中文翻译:

在低剂量下对巨噬细胞中各种基于金属的纳米粒子的生物效应进行多层次分析
迄今为止,已经做出了巨大的努力来理解金属基纳米颗粒(MNP)的生物安全性。尽管如此,以前的大多数研究都集中在浓度不切实际的高浓度MNPs的特定不良后果上,与国家职业安全与健康研究所(NIOSH)的暴露阈值几乎没有关系,并且未能全面评估其毒性特征。为了应对这些挑战,我们在这里努力在低暴露剂量下多层次地分析巨噬细胞中各种普遍使用的MNP的危害作用。在这些剂量下,没有引起明显的细胞活力下降和细胞死亡。但是,即使在低浓度下,所有MNP都可以在细胞中启动细胞抗氧化剂防御系统,尽管程度不同且途径不同,如抗氧化酶和Nrf2信号转导的差异诱导所反映的。关于炎症,除nCeO外,稀土氧化物纳米材料(REO)2以NLRP3炎性小体依赖性方式大大增加了IL-1β分泌。相比之下,发现六个REOs AgNP-5nm,nFe 2 O 3,nFe 3 O 4和nZnO通过转录后调节来提高TNF-α的浓度。此外,除nCeO 2以外的所有MNP都极大地改变了细胞膜/细胞骨架的网状结构,但导致了不同的结果,细胞大小缩小,REO突出的突起数量减少,其他MNP突出的突起数量减少。因此,REO(例如nDy 2 O 3和nSm 2 O 3)巨噬细胞的吞噬功能受损,而其他MNP(例如AgNP-25nm和nZnO)则反过来增强了巨噬细胞的吞噬能力。这些MNP诱导的膜和细胞骨架网状结构的改变也会引起膜电位和钙离子通量的紊乱。总的来说,我们的数据概述了低暴露剂量下巨噬细胞中不同MNP的生物学效应,并破译了一个复杂的网络,该网络将多重平行的途径和过程与不同的不良后果联系起来。


























 京公网安备 11010802027423号
京公网安备 11010802027423号