Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-06-30 , DOI: 10.1016/j.tetlet.2018.06.069 Hui Shao , Xiu-Wu Huang , Lei Song , Wen-Ting Zhou , Guan-Ping Tang , Long Ye
|
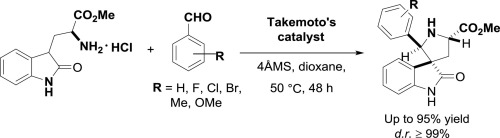
The organocatalyzed asymmetric Mannich-type spirocyclization was employed to access the spiro[pyrrolidin-3,3′-oxindoles], a biologically privileged ring structure, from readily available 2-oxotryptaphan methyl ester and aromatic aldehydes. The stereoselectivity of this cyclization are closely correlated with the organocatalyst and solvent used for the reaction. The best diastereoselectivity (d.r. ≥99%) was achieved using only 5% loading of the bifunctional organocatalyst, Takemoto’s thiourea, and dioxane as solvent, resulting in the formation of the the spirocycle with the (2′R, 3R) configuration. The yield could be increased up to 97% when excess of benzaldehyde was used to overcome its trimerization.
中文翻译:

通过有机催化不对称曼尼希型反应立体选择性合成螺[吡咯烷3,3'-羟吲哚]
利用有机催化的不对称曼尼希型螺环化反应从容易获得的2-氧代色氨酸甲基酯和芳香族醛类中获得螺环[吡咯烷烃-3,3'-羟吲哚],一种具有生物学优势的环结构。该环化的立体选择性与用于反应的有机催化剂和溶剂密切相关。使用双功能有机催化剂,竹本硫脲和二恶烷作为溶剂仅5%的负载量即可达到最佳非对映选择性(dr≥99%),从而形成具有(2'R,3R)构型的螺环。当使用过量的苯甲醛克服其三聚作用时,产率可增加至97%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号