Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-06-30 , DOI: 10.1016/j.tetlet.2018.06.063 Alex J Berkowitz 1, 2 , Rudolf G Abdelmessih 1 , Ryan P Murelli 1, 2
|
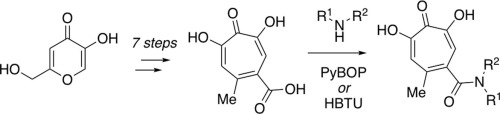
α-Hydroxytropolones (αHTs) are excellent metalloenzyme-inhibiting fragments that have been the basis for the development of potent inhibitors of various therapeutically important enzymes. The following manuscript describes a final-step amidation approach for αHT diversification. The method takes advantage of a scalable, chromatography-free synthesis of a carboxylic acid-appended αHT, and the present manuscript describes the conversion of this compound to eight amide-containing αHTs, three of which should be useful as chemical probes. This general strategy is expected to find widespread usage in both chemical biology and medicinal chemistry studies on αHTs.
中文翻译:

最后一步 α-羟基托酚酮多样化的酰胺化策略
α-羟基托酚酮 (αHT) 是优异的金属酶抑制片段,是开发各种重要治疗酶的有效抑制剂的基础。以下手稿描述了 αHT 多样化的最后一步酰胺化方法。该方法利用了羧酸附加αHT的可扩展、无色谱合成,本手稿描述了将该化合物转化为八种含酰胺的αHT,其中三种可用作化学探针。这一总体策略预计将在 αHT 的化学生物学和药物化学研究中得到广泛应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号