当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic Asymmetric Four‐Component [5+1+1+1] Cycloadditions via a Quintuple Cascade Process
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-07-19 , DOI: 10.1002/adsc.201800636 Wei Xiao 1 , Zhi Zhou 1 , Qian-Qian Yang 1 , Wei Du 1 , Ying-Chun Chen 1, 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-07-19 , DOI: 10.1002/adsc.201800636 Wei Xiao 1 , Zhi Zhou 1 , Qian-Qian Yang 1 , Wei Du 1 , Ying-Chun Chen 1, 2
Affiliation
|
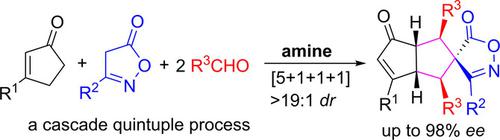
An asymmetric four‐component [5+1+1+1] formal cycloaddition reaction of 3‐substituted 2‐cyclopentenones, activated methylene nucleophiles and two molecules of aldehydes is developed under chiral primary aminocatalysis, producing a diversity of highly enantioenriched fused and spirocyclic frameworks with high molecular complexity. Mechanism studies indicate that the current reaction proceeds in a challenging cascade quintuple Knoevenagel condensation/Michael addition/retro‐Michael addition/Michael addition/Michael addition sequence via diverse aminocatalytic modes.
中文翻译:

通过五重级联过程的有机催化不对称四组分[5 + 1 + 1 + 1]环加成反应
在手性伯氨基催化条件下,发生了由3-取代的2-环戊烯酮,活化的亚甲基亲核试剂和两个醛类分子组成的不对称四组分[5 + 1 + 1 + 1]正式环加成反应,产生了多种高度对映体富集的稠合和螺环骨架分子复杂性高。机理研究表明,当前的反应以具有挑战性的级联五元Knoevenagel缩合/ Michael加成/复古Michael加成/ Michael加成/ Michael加成序列通过多种氨基催化模式进行。
更新日期:2018-07-19
中文翻译:

通过五重级联过程的有机催化不对称四组分[5 + 1 + 1 + 1]环加成反应
在手性伯氨基催化条件下,发生了由3-取代的2-环戊烯酮,活化的亚甲基亲核试剂和两个醛类分子组成的不对称四组分[5 + 1 + 1 + 1]正式环加成反应,产生了多种高度对映体富集的稠合和螺环骨架分子复杂性高。机理研究表明,当前的反应以具有挑战性的级联五元Knoevenagel缩合/ Michael加成/复古Michael加成/ Michael加成/ Michael加成序列通过多种氨基催化模式进行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号