Chemical Physics ( IF 2.3 ) Pub Date : 2018-06-28 , DOI: 10.1016/j.chemphys.2018.06.022 Saeid Azizian , Setareh Eris , Lee D. Wilson
|
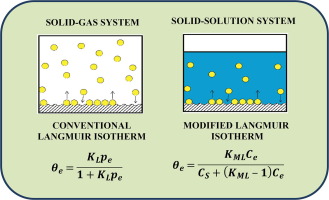
One of the first reported equations to describe adsorption at equilibrium is the Langmuir model (J. Am. Chem. Soc.1918, 40, 1361-1403). Initially, the model was used to describe adsorption from the gas phase, along with adsorption from the liquid phase. Herein, the shortcomings of the Langmuir model for adsorption processes from liquid phase are revealed. This study reports a revised form of the Langmuir isotherm model that provides further insight on the nature of adsorption and desorption processes in condensed phases. This approach is anticipated to provide a theoretical background for universal calculation of the adsorption thermodynamic parameters. We also provide an overview of the methods that use the Langmuir equilibrium constant for calculation of thermodynamic parameters of adsorption and the reasons for their limitations are highlighted.
中文翻译:

重新评估具有百年历史的Langmuir等温线以模拟溶液中的吸附现象
最早描述平衡吸附的方程式之一是Langmuir模型(J.Am.Chem.Soc.1918,40,1361-1403)。最初,该模型用于描述气相的吸附以及液相的吸附。在此,揭示了Langmuir模型在液相吸附过程中的缺点。这项研究报告了Langmuir等温线模型的修订形式,该模型提供了有关凝结相中吸附和解吸过程的性质的进一步信息。预期该方法将为通用计算吸附热力学参数提供理论背景。我们还概述了使用Langmuir平衡常数计算吸附热力学参数的方法,并着重说明了其局限性的原因。


























 京公网安备 11010802027423号
京公网安备 11010802027423号