Journal of Solid State Chemistry ( IF 3.3 ) Pub Date : 2018-06-13 , DOI: 10.1016/j.jssc.2018.06.014 Kezhou Yan , Yanxia Guo , Dandan Liu , Zhibin Ma , Fangqin Cheng
|
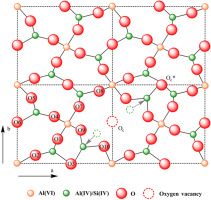
Mullite as one of the major aluminum-containing minerals in pulverized coal fly ash (PCFA) has a stable crystal structure, resulting in the difficulty in aluminum extraction from PCFA. Na2CO3-assisted thermal activation of PCFA is an efficient approach to activate the reactivity of mullite in PCFA. A clear understanding of the decomposition and transformation mechanism of mullite calcined with Na2CO3 is of great significance to clarify the activation mechanism of PCFA and the further process optimization. In this work, the thermal decomposition behavior, the phase transformation and the microstructure change were investigated in detail using thermo-gravimetric and differential scanning calorimetric (TG-DSC), X-ray diffraction (XRD) and magic angle spinning nuclear magnetic resonance (MAS-NMR). The results showed that the phase transformation process was mullite firstly converting into sodium aluminum silicate (SAS, Na1+xAl1+xSi1-xO4, 0 < x < 1) at< 800 °C and then to other minerals with higher structure stability, such as sodium aluminate and sodium silicate, with the increasing of Na2CO3 amount at> 800 °C. The reaction mechanism could be interpreted in terms of the combination change of different oxygen coordination. During the calcination, the Na2O decomposed from Na2CO3 would enter in mullite through filling oxygen vacancies and then react with the oxygen atoms around the AlVI, resulting in the formation of the AlO4 in SAS. Further increase in the calcination temperature and the Na2CO3 amount, the transforming from mullite to SAS would be obviously enhanced. When the temperature was> 800 °C, the residual AlO6 originated from mullite was further transformed into AlO4 with the larger amount of Na2CO3. Meanwhile, the bridging oxygen bonds between AlO4 and SiO4 in SAS were broken by the attacking of Na2O. Subsequently, all the obtained AlO4 and SiO4 as well as Na+ ion could be further recombined in a specific manner to transform into sodium aluminates (AlO4-AlO4), sodium silicates (SiO4-SiO4) and sodium aluminosilicates (AlO4-SiO4).
中文翻译:

碳酸钠作用下莫来石的热分解和转化机理
莫来石粉是粉煤灰(PCFA)中主要的含铝矿物之一,具有稳定的晶体结构,导致难以从PCFA中提取铝。Na 2 CO 3辅助的PCFA热活化是激活莫来石在PCFA中反应性的有效方法。清楚了解Na 2 CO 3煅烧莫来石的分解和转化机理对于阐明PCFA的活化机理和进一步的工艺优化具有重要意义。在这项工作中,使用热重和差示扫描量热法(TG-DSC),X射线衍射(XRD)和魔角旋转核磁共振(MAS)详细研究了热分解行为,相变和微观结构变化。 -NMR)。结果表明,相变过程中的莫来石首先转换成铝硅酸钠(SAS,钠1+ X的Al 1+ X的Si 1- X Ò 4,0 < X <1)在<800°C时,然后随着> 800°C的Na 2 CO 3含量增加,生成具有更高结构稳定性的其他矿物,例如铝酸钠和硅酸钠。反应机理可以根据不同氧配位的组合变化来解释。在煅烧期间,从Na 2 CO 3分解的Na 2 O将通过填充氧空位进入莫来石,然后与Al VI周围的氧原子反应,导致在SAS中形成AlO 4。煅烧温度和Na 2 CO 3进一步升高从数量上看,莫来石向SAS的转化将明显增强。当温度> 800℃时,源自莫来石的残余AlO 6进一步转化为具有较大Na 2 CO 3含量的AlO 4。同时,由于Na 2 O的侵蚀,SAS中的AlO 4和SiO 4之间的桥连氧键被破坏。随后,所有获得的AlO 4和SiO 4以及Na +离子都可以以特定的方式进一步重组以转化。分为铝酸钠(AlO 4 -AlO 4),硅酸钠(SiO 4-SiO 4)和铝硅酸钠(AlO 4 -SiO 4)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号