Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-06-28 , DOI: 10.1016/j.jcat.2018.06.007 Jin-Bao Peng , Hui-Qing Geng , Wei Wang , Xinxin Qi , Jun Ying , Xiao-Feng Wu
|
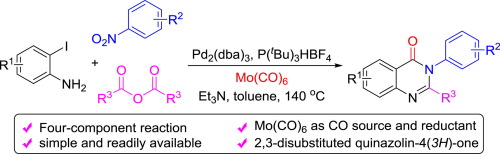
A palladium-catalyzed four-component carbonylative cyclization reaction for the synthesis of 2,3-disubstituted quinazolin-4(3H)-ones has been developed. A range of different 2,3-disubstituted quinazolin-4(3H)-one derivatives were prepared in moderate to good yields employing simple and readily accessible 2-iodoanilines, nitro compounds and acid anhydrides as the synthetic precursors. Mo(CO)6 acted both as a solid CO source and a reductant. Notably, methaqualone as a sedative and hypnotic medication can be prepared easily in 68% yield (4b) under our conditions as well.
中文翻译:

钯催化的四组分羰基合成2,3-二取代的喹唑啉-4(3H)-ones:方便的甲喹酮制备
已经开发了钯催化的四组分羰基环化反应,用于合成2,3-二取代的喹唑啉-4(3H)-one。使用简单且易于获得的2-碘苯胺,硝基化合物和酸酐作为合成前体,以中等至良好的产率制备了一系列不同的2,3-二取代的喹唑啉-4(3H)-one衍生物。Mo(CO)6既充当固体CO源又充当还原剂。值得注意的是,在我们的条件下,甲喹酮作为镇静剂和催眠药也可以很容易地制备,收率为68%(4b)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号