当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaluation of topologically distinct constrained antimicrobial peptides with broad-spectrum antimicrobial activity†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1039/c8ob00483h Fang Yuan 1, 2, 3, 4, 5 , Yuan Tian 3, 4, 6, 7, 8 , Weirong Qin 4, 5, 9, 10, 11 , Jingxu Li 4, 5, 9, 10, 11 , Dan Yang 4, 5, 9, 10, 11 , Bingchuan Zhao 4, 5, 9, 10, 11 , Feng Yin 4, 5, 9, 10, 11 , Zigang Li 4, 5, 9, 10, 11
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1039/c8ob00483h Fang Yuan 1, 2, 3, 4, 5 , Yuan Tian 3, 4, 6, 7, 8 , Weirong Qin 4, 5, 9, 10, 11 , Jingxu Li 4, 5, 9, 10, 11 , Dan Yang 4, 5, 9, 10, 11 , Bingchuan Zhao 4, 5, 9, 10, 11 , Feng Yin 4, 5, 9, 10, 11 , Zigang Li 4, 5, 9, 10, 11
Affiliation
|
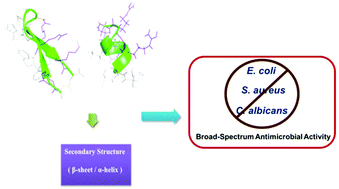
Antimicrobial peptides (AMPs) are short cationic peptides with a high affinity for membranes and emerged as a promising therapeutic approach with potential for treating infectious diseases. Chemical stabilization of short peptides proved to be a successful approach for enhancing their bio-physical properties. Herein, we designed and synthesized a panel of conformationally constrained antimicrobial peptides with either α-helical or β-hairpin conformation using templating strategies. These synthetic short constrained peptides possess different topological distributions of hydrophobic and hydrophilic residues and displayed distinct antimicrobial activity. Notably, the conformationally constrained α-helical peptides displayed a faster internalization into the bacteria cells compared to their β-hairpin analogues. These synthetic short constrained peptides showed killing effects on a broad spectrum of microorganisms mainly through pore formation and membrane damage which provided a potentially promising skeleton for the next generation of stabilized antimicrobial peptides.
中文翻译:

评估具有广谱抗菌活性的拓扑学上不同的受限抗菌肽†
抗菌肽(AMPs)是对膜具有高度亲和力的短阳离子肽,是一种有前途的治疗方法,具有治疗传染病的潜力。短肽的化学稳定被证明是增强其生物物理特性的成功方法。在这里,我们使用模板策略设计和合成了一组具有α-螺旋或β-发夹构象的构象受限的抗菌肽。这些合成的短约束肽具有疏水和亲水残基的不同拓扑分布,并显示出独特的抗菌活性。值得注意的是,与其β-发夹类似物相比,构象受约束的α-螺旋肽显示出更快的内在化进入细菌细胞。
更新日期:2018-06-27
中文翻译:

评估具有广谱抗菌活性的拓扑学上不同的受限抗菌肽†
抗菌肽(AMPs)是对膜具有高度亲和力的短阳离子肽,是一种有前途的治疗方法,具有治疗传染病的潜力。短肽的化学稳定被证明是增强其生物物理特性的成功方法。在这里,我们使用模板策略设计和合成了一组具有α-螺旋或β-发夹构象的构象受限的抗菌肽。这些合成的短约束肽具有疏水和亲水残基的不同拓扑分布,并显示出独特的抗菌活性。值得注意的是,与其β-发夹类似物相比,构象受约束的α-螺旋肽显示出更快的内在化进入细菌细胞。



























 京公网安备 11010802027423号
京公网安备 11010802027423号