当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating the Effects of Metal Complexation on Morphological and Rheological Properties of Polymer Solutions by a Dissipative Particle Dynamics Model
Macromolecules ( IF 5.5 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.macromol.8b00493 Kolattukudy P. Santo 1 , Aleksey Vishnyakov 1 , Ravish Kumar 1 , Alexander V. Neimark 1
Macromolecules ( IF 5.5 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.macromol.8b00493 Kolattukudy P. Santo 1 , Aleksey Vishnyakov 1 , Ravish Kumar 1 , Alexander V. Neimark 1
Affiliation
|
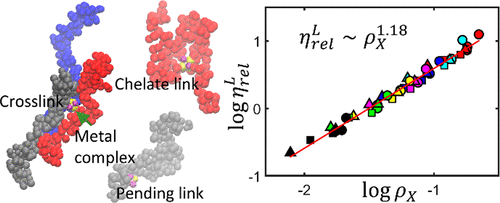
When a salt is added to a polymer solution, metal cations may coordinate with polymer ligands forming interchain and intrachain links. Metal coordination leads to drastic changes of polymer morphology, formation of clusters, and, ultimately, a sol–gel transition that affect the solution rheology. Although metal coordination is ubiquitous in polymeric systems, the physical mechanisms of coordination-induced morphological and rheological changes are still poorly understood due to the multiscale nature of this phenomenon. Here, we propose a coarse-grained dissipative particle dynamics (DPD) model to study morphological and rheological properties of concentrated solutions of polymers in the presence of multivalent cations that can coordinate the polymer ligands. The coordinating metal is introduced as a 3D complex of planar, tetrahedral, or octahedral geometry with the central DPD bead representing the metal cation surrounded at the vertices by either four or six dummy beads representing coordination sites, some of which are occupied by counterions to provide electroneutrality of the complex. Coordination is modeled as the dynamic formation and dissociation of a reversible link between the vacant coordination site and a ligand described by the Morse potential. The proposed model is applied to study the specifics of the equilibrium morphology and shearing flow in polyvinylpyrrolidone–dimethylformamide solutions in the presence of metal chlorides. Coordination leads to interchain and intrachain cross-links as well as to metal cations grafted onto polymer chains by a single link. The interchain cross-links induce a sol–gel transition to a weak gel phase as the metal concentration increases. Because of the reversible nature of interchain cross-links, the weak gel phase behaves as a viscoelastic fluid, the viscosity of which gradually increases with the metal concentration and decreases as the shear rate increases. The change of viscosity due to interchain coordination cross-links scales with the interchain cross-link density and the metal concentration according to the power law with the exponent ν ≈ 1.15. The influence of the grafted metal atoms on the viscosity is found to be much weaker, while the effect of the intrachain cross-links is found to be negligible. The simulation results are in qualitative agreement with available literature data. The proposed DPD model provides a physical insight into the morphological features of polymer solutions in the presence of multivalent slats and can be extended to other coordinating systems such as metal-substituted polyelectrolytes.
中文翻译:

通过耗散粒子动力学模型阐明金属络合对聚合物溶液形态和流变性质的影响
当将盐添加到聚合物溶液中时,金属阳离子可能与形成链间和链内连接的聚合物配体配位。金属配位导致聚合物形态的急剧变化,团簇的形成,并最终导致影响溶液流变性的溶胶-凝胶转变。尽管金属配位在聚合物体系中无处不在,但由于这种现象的多尺度性质,对配位诱导的形态和流变变化的物理机理仍然知之甚少。在这里,我们提出了一种粗颗粒的耗散粒子动力学(DPD)模型,以研究在可以配位聚合物配体的多价阳离子存在下,聚合物浓缩溶液的形态和流变特性。配位金属以平面,四面体,或八面体几何形状,中心DPD磁珠代表金属阳离子,在顶点处被四个或六个虚拟磁珠围绕,这些虚拟磁珠代表配位点,其中一些被反离子占据以提供络合物的电中性。配位被建模为空位配位点与摩尔斯电势所描述的配体之间可逆连接的动态形成和解离。所提出的模型用于研究在存在金属氯化物的情况下聚乙烯吡咯烷酮-二甲基甲酰胺溶液的平衡形态和剪切流的特性。配位导致链间和链内交联以及通过单个链接枝到聚合物链上的金属阳离子。随着金属浓度的增加,链间交联导致溶胶-凝胶转变为弱凝胶相。由于链间交联的可逆性质,弱凝胶相表现为粘弹性流体,其粘度随金属浓度而逐渐增加,并随着剪切速率的增加而降低。链间配位交联引起的粘度变化与链间交联密度和金属浓度成正比,与幂律成正比,指数ν≈1.15。发现接枝的金属原子对粘度的影响要弱得多,而发现链内交联的影响可以忽略。仿真结果与现有文献数据在质量上吻合。
更新日期:2018-06-27
中文翻译:

通过耗散粒子动力学模型阐明金属络合对聚合物溶液形态和流变性质的影响
当将盐添加到聚合物溶液中时,金属阳离子可能与形成链间和链内连接的聚合物配体配位。金属配位导致聚合物形态的急剧变化,团簇的形成,并最终导致影响溶液流变性的溶胶-凝胶转变。尽管金属配位在聚合物体系中无处不在,但由于这种现象的多尺度性质,对配位诱导的形态和流变变化的物理机理仍然知之甚少。在这里,我们提出了一种粗颗粒的耗散粒子动力学(DPD)模型,以研究在可以配位聚合物配体的多价阳离子存在下,聚合物浓缩溶液的形态和流变特性。配位金属以平面,四面体,或八面体几何形状,中心DPD磁珠代表金属阳离子,在顶点处被四个或六个虚拟磁珠围绕,这些虚拟磁珠代表配位点,其中一些被反离子占据以提供络合物的电中性。配位被建模为空位配位点与摩尔斯电势所描述的配体之间可逆连接的动态形成和解离。所提出的模型用于研究在存在金属氯化物的情况下聚乙烯吡咯烷酮-二甲基甲酰胺溶液的平衡形态和剪切流的特性。配位导致链间和链内交联以及通过单个链接枝到聚合物链上的金属阳离子。随着金属浓度的增加,链间交联导致溶胶-凝胶转变为弱凝胶相。由于链间交联的可逆性质,弱凝胶相表现为粘弹性流体,其粘度随金属浓度而逐渐增加,并随着剪切速率的增加而降低。链间配位交联引起的粘度变化与链间交联密度和金属浓度成正比,与幂律成正比,指数ν≈1.15。发现接枝的金属原子对粘度的影响要弱得多,而发现链内交联的影响可以忽略。仿真结果与现有文献数据在质量上吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号