当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electronic and Steric Tuning of Catalytic H2 Evolution by Cobalt Complexes with Pentadentate Polypyridyl-Amine Ligands
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-27 , DOI: 10.1021/jacs.8b05108 Ping Wang 1 , Guangchao Liang 2 , M. Ramana Reddy 1 , Melissa Long 1 , Kandria Driskill 3 , Christian Lyons 4 , Bruno Donnadieu 2 , John C. Bollinger 5 , Charles Edwin Webster 2 , Xuan Zhao 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-27 , DOI: 10.1021/jacs.8b05108 Ping Wang 1 , Guangchao Liang 2 , M. Ramana Reddy 1 , Melissa Long 1 , Kandria Driskill 3 , Christian Lyons 4 , Bruno Donnadieu 2 , John C. Bollinger 5 , Charles Edwin Webster 2 , Xuan Zhao 1
Affiliation
|
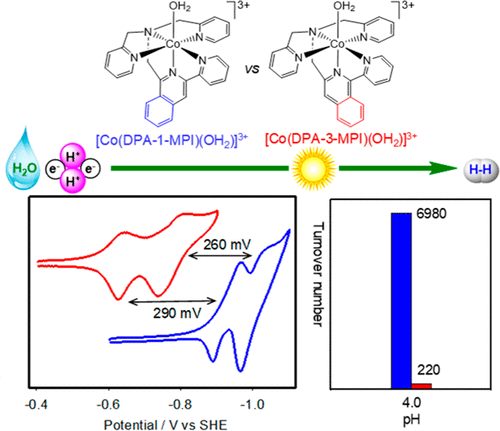
Structural modifications of molecular cobalt catalysts have provided important insights into the structure-function relationship for the hydrogen evolution reaction. We have shown that replacement of equatorial pyridines with more basic and conjugate isoquinoline groups of a pentadentate ligand results in lower overpotential and higher catalytic activity for electro- and photolytic H2 production in aqueous solutions. To fully understand the electronic and steric effects of the axial group that lies trans to the proposed cobalt hydride intermediate, isoquinoline groups were introduced in two new pentadentate ligands, N, N-bis(2-pyridinylmethyl)[3-(2-pyridinyl)isoquinoline)]-1-methanamine (DPA-1-MPI) and N, N-bis(2-pyridinylmethyl)[1-(2-pyridinyl)-isoquinoline)]-3-methanamine (DPA-3-MPI). Despite a slight structural difference of the introduced isoquinoline group, the resulting cobalt complexes display drastic changes in their electro- and photochemical properties. There are positive shifts of 290 and 260 mV, respectively, for the CoII/CoI and CoIII-H/CoII-H couples from [Co(DPA-1-MPI)(H2O)](PF6)3 to [Co(DPA-3-MPI)(H2O)](PF6)3, with the former being ∼32 times as active as the latter in photocatalytic H2 production. Density functional theory (DFT) calculations show that the protonation of CoI to yield the CoIII-H species is energetically more favorable for [Co(DPA-1-MPI)(H2O)](PF6)3 than that of [Co(DPA-3-MPI)(H2O)](PF6)3. Both experimental results and DFT computations suggest that the presence of a planar conjugate bipyridyl unit or its isoquinoline derivative is a key feature for stabilizing low valent CoI species toward proton binding. The incorporation of an electron-donating group trans to the proposed Co-H species also facilitates proton binding and H-H bond formation, which is proposed to occur by the heterolytic coupling of CoII-H species. The overall catalytic H2 evolution is presented as the modified electron transfer (E)-proton transfer (C)-electron transfer (E)-proton transfer (C) (mod-ECEC) pathway. This study provides important new insight into the electronic and steric factors controlling catalytic H2 production by Co complexes with pentadentate ligands.
中文翻译:

钴配合物与五齿多吡啶胺配体催化 H2 释放的电子和空间调节
分子钴催化剂的结构改性为析氢反应的结构-功能关系提供了重要的见解。我们已经表明,用五齿配体的更多碱性和共轭异喹啉基团替换赤道吡啶会导致较低的过电位和较高的催化活性,用于水溶液中的电和光解 H2 生产。为了充分理解与所提出的氢化钴中间体反式的轴基的电子和空间效应,在两个新的五齿配体中引入了异喹啉基团,N,N-双(2-吡啶基甲基)[3-(2-吡啶基)异喹啉)]-1-甲胺 (DPA-1-MPI) 和 N, N-双(2-吡啶基甲基)[1-(2-吡啶基)-异喹啉)]-3-甲胺 (DPA-3-MPI)。尽管引入的异喹啉基团在结构上略有不同,但由此产生的钴配合物在其电化学和光化学性质方面显示出巨大的变化。对于 CoII/CoI 和 CoIII-H/CoII-H 对,从 [Co(DPA-1-MPI)(H2O)](PF6)3 到 [Co(DPA- 3-MPI)(H2O)](PF6)3,前者在光催化制氢中的活性是后者的 32 倍。密度泛函理论 (DFT) 计算表明,CoI 质子化以产生 CoIII-H 物种在能量上对 [Co(DPA-1-MPI)(H2O)](PF6)3 比 [Co(DPA- 3-MPI)(H2O)](PF6)3。实验结果和 DFT 计算表明,平面共轭联吡啶单元或其异喹啉衍生物的存在是稳定低价 CoI 物质与质子结合的关键特征。将给电子基团反式引入所提出的 Co-H 物种也促进了质子结合和 HH 键的形成,这被认为是通过 CoII-H 物种的异裂偶联发生的。整体催化 H2 演化表现为改进的电子转移 (E)-质子转移 (C)-电子转移 (E)-质子转移 (C) (mod-ECEC) 途径。这项研究为控制 Co 与五齿配体的配合物催化制氢的电子和空间因素提供了重要的新见解。将给电子基团反式引入所提出的 Co-H 物种也促进了质子结合和 HH 键的形成,这被认为是通过 CoII-H 物种的异裂偶联发生的。整体催化 H2 演化表现为改进的电子转移 (E)-质子转移 (C)-电子转移 (E)-质子转移 (C) (mod-ECEC) 途径。这项研究为控制 Co 与五齿配体的配合物催化制氢的电子和空间因素提供了重要的新见解。将给电子基团反式引入所提出的 Co-H 物种也促进了质子结合和 HH 键的形成,这被认为是通过 CoII-H 物种的异裂偶联发生的。整体催化 H2 演化表现为改进的电子转移 (E)-质子转移 (C)-电子转移 (E)-质子转移 (C) (mod-ECEC) 途径。这项研究为控制 Co 与五齿配体的配合物催化制氢的电子和空间因素提供了重要的新见解。
更新日期:2018-06-27
中文翻译:

钴配合物与五齿多吡啶胺配体催化 H2 释放的电子和空间调节
分子钴催化剂的结构改性为析氢反应的结构-功能关系提供了重要的见解。我们已经表明,用五齿配体的更多碱性和共轭异喹啉基团替换赤道吡啶会导致较低的过电位和较高的催化活性,用于水溶液中的电和光解 H2 生产。为了充分理解与所提出的氢化钴中间体反式的轴基的电子和空间效应,在两个新的五齿配体中引入了异喹啉基团,N,N-双(2-吡啶基甲基)[3-(2-吡啶基)异喹啉)]-1-甲胺 (DPA-1-MPI) 和 N, N-双(2-吡啶基甲基)[1-(2-吡啶基)-异喹啉)]-3-甲胺 (DPA-3-MPI)。尽管引入的异喹啉基团在结构上略有不同,但由此产生的钴配合物在其电化学和光化学性质方面显示出巨大的变化。对于 CoII/CoI 和 CoIII-H/CoII-H 对,从 [Co(DPA-1-MPI)(H2O)](PF6)3 到 [Co(DPA- 3-MPI)(H2O)](PF6)3,前者在光催化制氢中的活性是后者的 32 倍。密度泛函理论 (DFT) 计算表明,CoI 质子化以产生 CoIII-H 物种在能量上对 [Co(DPA-1-MPI)(H2O)](PF6)3 比 [Co(DPA- 3-MPI)(H2O)](PF6)3。实验结果和 DFT 计算表明,平面共轭联吡啶单元或其异喹啉衍生物的存在是稳定低价 CoI 物质与质子结合的关键特征。将给电子基团反式引入所提出的 Co-H 物种也促进了质子结合和 HH 键的形成,这被认为是通过 CoII-H 物种的异裂偶联发生的。整体催化 H2 演化表现为改进的电子转移 (E)-质子转移 (C)-电子转移 (E)-质子转移 (C) (mod-ECEC) 途径。这项研究为控制 Co 与五齿配体的配合物催化制氢的电子和空间因素提供了重要的新见解。将给电子基团反式引入所提出的 Co-H 物种也促进了质子结合和 HH 键的形成,这被认为是通过 CoII-H 物种的异裂偶联发生的。整体催化 H2 演化表现为改进的电子转移 (E)-质子转移 (C)-电子转移 (E)-质子转移 (C) (mod-ECEC) 途径。这项研究为控制 Co 与五齿配体的配合物催化制氢的电子和空间因素提供了重要的新见解。将给电子基团反式引入所提出的 Co-H 物种也促进了质子结合和 HH 键的形成,这被认为是通过 CoII-H 物种的异裂偶联发生的。整体催化 H2 演化表现为改进的电子转移 (E)-质子转移 (C)-电子转移 (E)-质子转移 (C) (mod-ECEC) 途径。这项研究为控制 Co 与五齿配体的配合物催化制氢的电子和空间因素提供了重要的新见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号