当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed β-Arylation of Amide via Primary sp3C–H Activation
Organometallics ( IF 2.8 ) Pub Date : 2018-06-27 , DOI: 10.1021/acs.organomet.8b00325 Ren Zhao 1 , Wenjun Lu 1
Organometallics ( IF 2.8 ) Pub Date : 2018-06-27 , DOI: 10.1021/acs.organomet.8b00325 Ren Zhao 1 , Wenjun Lu 1
Affiliation
|
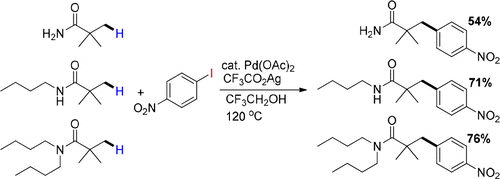
A β-arylation of primary sp3C–H bonds on simple amides such as pivalamides with aryl iodides/CF3CO2Ag has been established successfully at 120 °C in a Pd(OAc)2 (catalyst)/CF3CH2OH (solvent) system. Pivalamides including tBuCONH2, tBuCONHR, and tBuCONR2 undergo the arylations smoothly to afford β-aryl pivalamides in moderate to good yields. Various aryl iodides are available bearing either electron-donating or electron-withdrawing substituted groups in the coupling reactions.
中文翻译:

钯通过主sp 3 C–H活化催化酰胺的β-芳基化
在120°C的Pd(OAc)2(催化剂)/ CF 3 CH 2中成功建立了简单酰胺(如新戊酰胺)上的初生sp 3 C–H键与芳基碘化物/ CF 3 CO 2 Ag的β-芳基化作用。OH(溶剂)体系。包括t BuCONH 2,t BuCONHR和t BuCONR 2在内的新戊酰胺平稳地进行芳基化反应,以中等至良好的收率得到β-芳基新戊酰胺。可获得各种在偶联反应中带有给电子或吸电子取代基的芳基碘化物。
更新日期:2018-06-28
中文翻译:

钯通过主sp 3 C–H活化催化酰胺的β-芳基化
在120°C的Pd(OAc)2(催化剂)/ CF 3 CH 2中成功建立了简单酰胺(如新戊酰胺)上的初生sp 3 C–H键与芳基碘化物/ CF 3 CO 2 Ag的β-芳基化作用。OH(溶剂)体系。包括t BuCONH 2,t BuCONHR和t BuCONR 2在内的新戊酰胺平稳地进行芳基化反应,以中等至良好的收率得到β-芳基新戊酰胺。可获得各种在偶联反应中带有给电子或吸电子取代基的芳基碘化物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号