当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Naturally Occurring Disease-Related Mutations in the 40–57 Ω-Loop of Human Cytochrome c Control Triggering of the Alkaline Isomerization
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00520 Oliver M. Deacon 1 , Dimitri A. Svistunenko 1 , Geoffrey R. Moore 2 , Michael T. Wilson 1 , Jonathan A.R. Worrall 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00520 Oliver M. Deacon 1 , Dimitri A. Svistunenko 1 , Geoffrey R. Moore 2 , Michael T. Wilson 1 , Jonathan A.R. Worrall 1
Affiliation
|
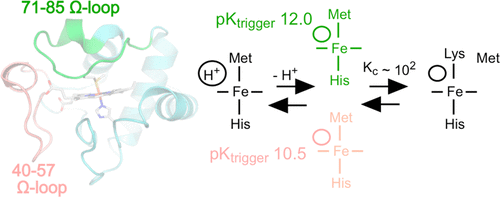
Naturally occurring mutations found in one of the two Ω-loop substructures in human cytochrome c are associated with low blood platelet count (thrombocytopenia). Both Ω-loops participate in the formation of conformers associated with cytochrome c peroxidase activity and apoptotic function. At alkaline pH values, the Met80 ligand to the ferric heme iron dissociates, and a lysine residue in the 71–85 Ω-loop coordinates to the iron. The alkaline isomerization has been the focus of extensive kinetic studies, and it is established that a deprotonation triggers the release of the Met80 ligand (pKtrigger). A second deprotonation stabilizes a pentacoordinate heme form (pKa2). In this study, site-directed variants at the 41 and 48 positions in the 40–57 Ω-loop and at the 81 and 83 positions in the 71–85 Ω-loop reveal that conformational transitions in the 71–85 Ω-loop, leading to the alkaline or peroxidatic conformers, are controlled by the 40–57 Ω-loop. We find that the variants causing thrombocytopenia, G41S and Y48H, lower the pKtrigger and increase pKa2. Our results are presented in a mechanistic framework, depicted by a cube, that accounts for the pH dependencies of the equilibrium and kinetic parameters governing the alkaline transition of the native protein and Ω-loop variants. The data are most consistent with the trigger for Met80 replacement by a lysine being a deprotonation within a hydrogen bonded unit that links the two Ω-loops rather than an individual group. Such a proposal aligns with the entatic contribution made by the same unit in controlling the Met80–Fe(III) bond strength.
中文翻译:

自然发生的与疾病相关的突变,在人类细胞色素c的40-57Ω环中引发碱性异构化
在人类细胞色素c的两个Ω环亚结构之一中发现的自然发生的突变与血小板计数低(血小板减少)有关。两个Ω环均参与与细胞色素c过氧化物酶活性和凋亡功能相关的构象异构体的形成。在碱性pH值下,血红素铁的Met80配体解离,并且71-85Ω环中的赖氨酸残基与铁形成坐标。碱性异构化一直是广泛动力学研究的重点,并且已确定去质子化可触发Met80配体的释放(p K触发)。第二次去质子稳定了五配位血红素形式(p K a2)。在这项研究中,定点变异在40-57Ω环的41和48位以及71-85Ω环的81和83位揭示了71-85Ω环的构象转变,导致呈碱性或过氧化物构象的分子,由40–57Ω环控制。我们发现引起血小板减少症,G41S和Y48H的变异体降低了p K触发并增加了p K a2。我们的结果显示在一个机械结构的框架中,用立方体表示,该框架说明了平衡的pH依赖性以及控制天然蛋白质和Ω-loop变体的碱性转变的动力学参数。数据与赖氨酸是Met80替代触发因素最一致,赖氨酸是连接两个Ω环而不是单个基团的氢键合单元内的去质子作用。这样的提议与同一单元在控制Met80-Fe(III)结合强度方面所做的积极贡献相吻合。
更新日期:2018-06-27
中文翻译:

自然发生的与疾病相关的突变,在人类细胞色素c的40-57Ω环中引发碱性异构化
在人类细胞色素c的两个Ω环亚结构之一中发现的自然发生的突变与血小板计数低(血小板减少)有关。两个Ω环均参与与细胞色素c过氧化物酶活性和凋亡功能相关的构象异构体的形成。在碱性pH值下,血红素铁的Met80配体解离,并且71-85Ω环中的赖氨酸残基与铁形成坐标。碱性异构化一直是广泛动力学研究的重点,并且已确定去质子化可触发Met80配体的释放(p K触发)。第二次去质子稳定了五配位血红素形式(p K a2)。在这项研究中,定点变异在40-57Ω环的41和48位以及71-85Ω环的81和83位揭示了71-85Ω环的构象转变,导致呈碱性或过氧化物构象的分子,由40–57Ω环控制。我们发现引起血小板减少症,G41S和Y48H的变异体降低了p K触发并增加了p K a2。我们的结果显示在一个机械结构的框架中,用立方体表示,该框架说明了平衡的pH依赖性以及控制天然蛋白质和Ω-loop变体的碱性转变的动力学参数。数据与赖氨酸是Met80替代触发因素最一致,赖氨酸是连接两个Ω环而不是单个基团的氢键合单元内的去质子作用。这样的提议与同一单元在控制Met80-Fe(III)结合强度方面所做的积极贡献相吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号