当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Methanosarcina mazei MM2060 Gene Encodes a Bifunctional Kinase/Decarboxylase Enzyme Involved in Cobamide Biosynthesis
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00546 Norbert K Tavares 1 , Carmen L Zayas 2 , Jorge C Escalante-Semerena 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00546 Norbert K Tavares 1 , Carmen L Zayas 2 , Jorge C Escalante-Semerena 1
Affiliation
|
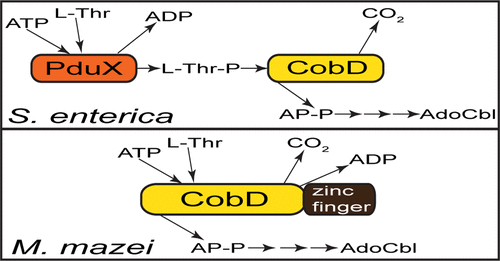
Cobamides (Cbas) are synthesized by many archaea, but some aspects of Cba biosynthesis in these microorganisms remain unclear. Here, we demonstrate that open reading frame MM2060 in the archaeum Methanosarcina mazei strain Gö1 encodes a bifunctional enzyme with l-threonine-O-3-phosphate (l-Thr-P) decarboxylase (EC 4.1.1.81) and l-Thr kinase activities (EC 2.7.1.177). In Salmonella enterica, where Cba biosynthesis has been extensively studied, the activities mentioned above are encoded by separate genes, namely, cobD and pduX, respectively. The activities associated with the MM2060 protein (MmCobD) were validated in vitro and in vivo. In vitro, MmCobD used ATP and l-Thr as substrates and generated ADP, l-Thr-P, and (R)-1-aminopropan-2-ol O-phosphate as products. Notably, MmCobD has a 111-amino acid C-terminal extension of unknown function, which contains a putative metal-binding motif. This C-terminal domain alone did not display activity either in vivo or in vitro. Although the C-terminal MmCobD domain was not required for l-Thr-P decarboxylase or l-Thr kinase activities in vivo, its absence negatively affected both activities. In vitro results suggested that this domain may have a regulatory or substrate-gating role. When purified under anoxic conditions, MmCobD displayed Michaelis–Menten kinetics and had a 1000-fold higher affinity for ATP and a catalytic efficiency 1300-fold higher than that of MmCobD purified under oxic conditions. To the best of our knowledge, MmCobD is the first example of a new class of l-Thr-P decarboxylases that also have l-Thr kinase activity. An archaeal protein with l-Thr kinase activity had not been identified prior to this work.
中文翻译:

Methanosarcina mazei MM2060 基因编码参与 Cobamide 生物合成的双功能激酶/脱羧酶
钴酰胺 (Cbas) 由许多古细菌合成,但这些微生物中 Cba 生物合成的某些方面仍不清楚。在这里,我们证明古菌Methanosarcina mazei菌株 Gö1 中的开放阅读框 MM2060 编码具有 l-苏氨酸-O -3-磷酸 (l-Thr-P) 脱羧酶 (EC 4.1.1.81) 和 l-Thr 激酶活性的双功能酶(EC 2.7.1.177)。在肠沙门氏菌中, Cba 生物合成已被广泛研究,上述活性分别由单独的基因编码,即cobD和pduX。与 MM2060 蛋白 ( Mm CobD)相关的活性已在体外和体内得到验证。在体外,Mm CobD以ATP和l-Thr为底物,生成ADP、l-Thr-P和( R )-1-氨基丙-2-醇O-磷酸作为产物。值得注意的是,Mm CobD 具有未知功能的 111 个氨基酸 C 端延伸,其中包含假定的金属结合基序。该C端结构域单独在体内或体外均不表现出活性。尽管体内L -Thr-P 脱羧酶或 L-Thr 激酶活性不需要C 端Mm CobD 结构域,但它的缺失会对这两种活性产生负面影响。体外结果表明该结构域可能具有调节或底物门控作用。在缺氧条件下纯化时,Mm CobD 表现出 Michaelis-Menten 动力学,与 ATP 的亲和力比在有氧条件下纯化的Mm CobD高 1000 倍,催化效率高 1300 倍。据我们所知,Mm CobD 是新型 l-Thr-P 脱羧酶的第一个例子,该酶也具有 l-Thr 激酶活性。在这项工作之前尚未鉴定出具有 l-Thr 激酶活性的古菌蛋白。
更新日期:2018-06-27
中文翻译:

Methanosarcina mazei MM2060 基因编码参与 Cobamide 生物合成的双功能激酶/脱羧酶
钴酰胺 (Cbas) 由许多古细菌合成,但这些微生物中 Cba 生物合成的某些方面仍不清楚。在这里,我们证明古菌Methanosarcina mazei菌株 Gö1 中的开放阅读框 MM2060 编码具有 l-苏氨酸-O -3-磷酸 (l-Thr-P) 脱羧酶 (EC 4.1.1.81) 和 l-Thr 激酶活性的双功能酶(EC 2.7.1.177)。在肠沙门氏菌中, Cba 生物合成已被广泛研究,上述活性分别由单独的基因编码,即cobD和pduX。与 MM2060 蛋白 ( Mm CobD)相关的活性已在体外和体内得到验证。在体外,Mm CobD以ATP和l-Thr为底物,生成ADP、l-Thr-P和( R )-1-氨基丙-2-醇O-磷酸作为产物。值得注意的是,Mm CobD 具有未知功能的 111 个氨基酸 C 端延伸,其中包含假定的金属结合基序。该C端结构域单独在体内或体外均不表现出活性。尽管体内L -Thr-P 脱羧酶或 L-Thr 激酶活性不需要C 端Mm CobD 结构域,但它的缺失会对这两种活性产生负面影响。体外结果表明该结构域可能具有调节或底物门控作用。在缺氧条件下纯化时,Mm CobD 表现出 Michaelis-Menten 动力学,与 ATP 的亲和力比在有氧条件下纯化的Mm CobD高 1000 倍,催化效率高 1300 倍。据我们所知,Mm CobD 是新型 l-Thr-P 脱羧酶的第一个例子,该酶也具有 l-Thr 激酶活性。在这项工作之前尚未鉴定出具有 l-Thr 激酶活性的古菌蛋白。



























 京公网安备 11010802027423号
京公网安备 11010802027423号