当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-Pot Sequential Kinetic Profiling of a Highly Reactive Manganese Catalyst for Ketone Hydroboration: Leveraging σ-Bond Metathesis via Alkoxide Exchange Steps
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-26 , DOI: 10.1021/jacs.8b05340 Vladislav Vasilenko 1 , Clemens K. Blasius 1 , Lutz H. Gade 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-26 , DOI: 10.1021/jacs.8b05340 Vladislav Vasilenko 1 , Clemens K. Blasius 1 , Lutz H. Gade 1
Affiliation
|
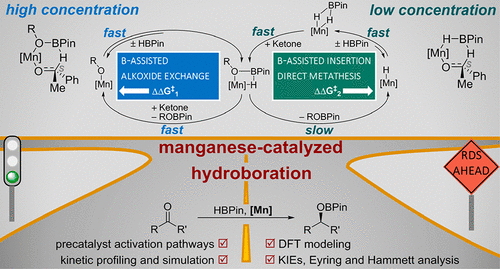
A comprehensive experimental and computational mechanistic study of the highly enantioselective hydroboration of ketones catalyzed by a manganese(II) alkyl boxmi pincer complex is reported. The catalyst operates at low catalyst loadings (down to 0.01 mol %) under very mild conditions (typically -40 °C) and facilitates the reduction of both aryl alkyl and dialkyl ketones with excellent selectivity (up to >95%ee). Catalyst activation pathways were investigated, demonstrating that a manganese(II) hydride and a manganese(II) alkoxide species are part of the catalytic cycle and can be generated via σ-bond metathesis of the alkyl precursor with the borane or by alcoholysis. Extensive kinetic experiments based on a "one-pot sequential kinetic profiling" approach under various conditions in combination with kinetic simulations reveal that two catalytic cycles are effective with this earth-abundant base metal catalyst: (i) a minor MnH/borane-mediated insertion cycle, in which the subsequent, product-releasing metathesis step is rate determining ( k m = 0.076 s-1), giving a background reaction, which is zeroth order in substrate concentrations, and (ii) a major MnOR/borane-based alkoxide exchange process, leveraging the high-barrier metathesis via the affiliation to an insertion step. The latter features non-integer reaction orders in both reagents due to a combination of an adduct formation step ( k a = 2.12 M-1 s-1, k -a = 0.49 s-1) and a substrate insertion step of comparable rates ( k ai = 3.74 M-1 s-1). The kinetic findings are underpinned by high-level density functional theory calculations of the mechanism, control experiments, and kinetic isotope effect/Hammett/Eyring analysis in different concentration regimes. The study highlights the role of a rigorous mechanistic understanding of homogeneous catalytic processes in 3d metals for rational catalyst discovery and optimization.
中文翻译:

用于酮硼氢化的高反应性锰催化剂的一锅连续动力学分析:通过醇盐交换步骤利用 σ-键复分解
报道了对由锰 (II) 烷基 boxmi 钳形配合物催化的酮的高度对映选择性硼氢化反应的综合实验和计算机制研究。该催化剂在非常温和的条件(通常为 -40 °C)下以低催化剂负载量(低至 0.01 mol%)运行,并以优异的选择性(高达 >95%ee)促进芳烷基和二烷基酮的还原。对催化剂活化途径进行了研究,表明氢化锰和锰(II)醇盐是催化循环的一部分,可以通过烷基前体与硼烷的 σ 键复分解或醇解产生。基于“一锅连续动力学分析”的广泛动力学实验 在各种条件下结合动力学模拟的方法表明,对于这种地球丰富的贱金属催化剂,两个催化循环是有效的:(i) 次要的 MnH/硼烷介导的插入循环,其中随后的释放产物的复分解步骤是速率确定(km = 0.076 s-1),给出底物浓度为零级的背景反应,以及(ii)主要的基于MnOR/硼烷的醇盐交换过程,通过与插入的隶属关系利用高阻隔复分解步。由于加合物形成步骤 (ka = 2.12 M-1 s-1, k -a = 0.49 s-1) 和速率相当的底物插入步骤 ( k ai = 3.74 M-1 s-1)。动力学研究结果得到了机制的高水平密度泛函理论计算、控制实验和不同浓度方案中的动力学同位素效应/哈米特/艾林分析的支持。该研究强调了对 3d 金属中均相催化过程的严格机械理解对于合理催化剂发现和优化的作用。
更新日期:2018-06-26
中文翻译:

用于酮硼氢化的高反应性锰催化剂的一锅连续动力学分析:通过醇盐交换步骤利用 σ-键复分解
报道了对由锰 (II) 烷基 boxmi 钳形配合物催化的酮的高度对映选择性硼氢化反应的综合实验和计算机制研究。该催化剂在非常温和的条件(通常为 -40 °C)下以低催化剂负载量(低至 0.01 mol%)运行,并以优异的选择性(高达 >95%ee)促进芳烷基和二烷基酮的还原。对催化剂活化途径进行了研究,表明氢化锰和锰(II)醇盐是催化循环的一部分,可以通过烷基前体与硼烷的 σ 键复分解或醇解产生。基于“一锅连续动力学分析”的广泛动力学实验 在各种条件下结合动力学模拟的方法表明,对于这种地球丰富的贱金属催化剂,两个催化循环是有效的:(i) 次要的 MnH/硼烷介导的插入循环,其中随后的释放产物的复分解步骤是速率确定(km = 0.076 s-1),给出底物浓度为零级的背景反应,以及(ii)主要的基于MnOR/硼烷的醇盐交换过程,通过与插入的隶属关系利用高阻隔复分解步。由于加合物形成步骤 (ka = 2.12 M-1 s-1, k -a = 0.49 s-1) 和速率相当的底物插入步骤 ( k ai = 3.74 M-1 s-1)。动力学研究结果得到了机制的高水平密度泛函理论计算、控制实验和不同浓度方案中的动力学同位素效应/哈米特/艾林分析的支持。该研究强调了对 3d 金属中均相催化过程的严格机械理解对于合理催化剂发现和优化的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号