当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-Based Membrane Electrodes Enable High-Rate Electrochemical Ammonia Recovery
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.est.8b01349 Dianxun Hou 1 , Arpita Iddya 2 , Xi Chen 1 , Mengyuan Wang 3 , Wenli Zhang 4 , Yifu Ding 3 , David Jassby 2 , Zhiyong Jason Ren 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.est.8b01349 Dianxun Hou 1 , Arpita Iddya 2 , Xi Chen 1 , Mengyuan Wang 3 , Wenli Zhang 4 , Yifu Ding 3 , David Jassby 2 , Zhiyong Jason Ren 1
Affiliation
|
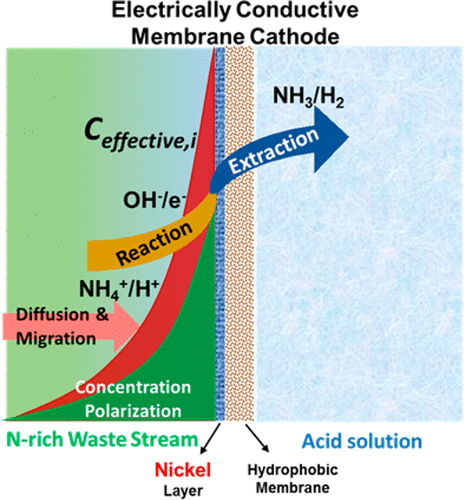
Wastewater contains significant amounts of nitrogen that can be recovered and valorized as fertilizers and chemicals. This study presents a new membrane electrode coupled with microbial electrolysis that demonstrates very efficient ammonia recovery from synthetic centrate. The process utilizes the electrical potential across electrodes to drive NH4+ ions toward the hydrophilic nickel top layer on a gas-stripping membrane cathode, which takes advantage of surface pH increase to realize spontaneous NH3 production and separation. Compared with a control configuration with conventionally separated electrode and hydrophobic membrane, the integrated membrane electrode showed 40% higher NH3–N recovery rate (36.2 ± 1.2 gNH3–N/m2/d) and 11% higher current density. The energy consumption was 1.61 ± 0.03 kWh/kgNH3–N, which was 20% lower than the control and 70–90% more efficient than competing electrochemical nitrogen recovery processes (5–12 kWh/kgNH3–N). Besides, the negative potential on membrane electrode repelled negatively charged organics and microbes thus reduced fouling. In addition to describing the system’s performance, we explored the underlying mechanisms governing the reactions, which confirmed the viability of this process for efficient wastewater–ammonia recovery. Furthermore, the nickel-based membrane electrode showed excellent water entry pressure (∼41 kPa) without leakage, which was much higher than that of PTFE/PDMS-based cathodes (∼1.8 kPa). The membrane electrode also showed superb flexibility (180° bend) and can be easily fabricated at low cost (<20 $/m2).
中文翻译:

镍基膜电极可实现高速率电化学氨的回收
废水中含有大量的氮,这些氮可以作为肥料和化学物质进行回收和增值。这项研究提出了一种新的膜电极,结合了微生物电解,证明了从合成精矿中非常有效地回收氨。该方法利用跨电极的电位将NH 4 +离子驱向气体剥离膜阴极上的亲水镍顶层,这利用表面pH值增加的优势实现了自发NH 3的 产生和分离。与具有常规分隔电极和疏水膜的控制配置相比,集成膜电极的NH 3 -N回收率高40%(36.2±1.2 gNH 3 -N / m2 / d)和更高的11%电流密度。能耗为1.61±0.03 kWh / kgNH 3 -N,比对照组低20%,比竞争的电化学氮回收工艺(5-12 kWh / kgNH 3)高70-90%–N)。此外,膜电极上的负电势排斥带负电的有机物和微生物,从而减少了结垢。除了描述系统的性能之外,我们还探索了控制反应的潜在机制,这证实了该过程对于高效废水-氨气回收的可行性。此外,镍基膜电极显示出优异的进水压力(〜41 kPa),无渗漏,远高于PTFE / PDMS基阴极(〜1.8 kPa)。膜电极还显示出极好的柔韧性(180°弯曲),并且可以很容易地以低成本(<20 $ / m 2)进行制造。
更新日期:2018-07-12
中文翻译:

镍基膜电极可实现高速率电化学氨的回收
废水中含有大量的氮,这些氮可以作为肥料和化学物质进行回收和增值。这项研究提出了一种新的膜电极,结合了微生物电解,证明了从合成精矿中非常有效地回收氨。该方法利用跨电极的电位将NH 4 +离子驱向气体剥离膜阴极上的亲水镍顶层,这利用表面pH值增加的优势实现了自发NH 3的 产生和分离。与具有常规分隔电极和疏水膜的控制配置相比,集成膜电极的NH 3 -N回收率高40%(36.2±1.2 gNH 3 -N / m2 / d)和更高的11%电流密度。能耗为1.61±0.03 kWh / kgNH 3 -N,比对照组低20%,比竞争的电化学氮回收工艺(5-12 kWh / kgNH 3)高70-90%–N)。此外,膜电极上的负电势排斥带负电的有机物和微生物,从而减少了结垢。除了描述系统的性能之外,我们还探索了控制反应的潜在机制,这证实了该过程对于高效废水-氨气回收的可行性。此外,镍基膜电极显示出优异的进水压力(〜41 kPa),无渗漏,远高于PTFE / PDMS基阴极(〜1.8 kPa)。膜电极还显示出极好的柔韧性(180°弯曲),并且可以很容易地以低成本(<20 $ / m 2)进行制造。


























 京公网安备 11010802027423号
京公网安备 11010802027423号