当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploration of the Effects of γ-Phosphate-Modified ATP Analogues on Histidine Kinase Autophosphorylation
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.biochem.8b00485 Olivia M. Chase 1 , Adeline Espinasse 1 , Kaelyn E. Wilke 2 , Erin E. Carlson 1, 2, 3, 4
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.biochem.8b00485 Olivia M. Chase 1 , Adeline Espinasse 1 , Kaelyn E. Wilke 2 , Erin E. Carlson 1, 2, 3, 4
Affiliation
|
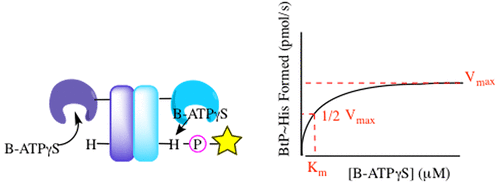
While two-component systems (TCSs), composed of a sensor histidine kinase (HK) and a response regulator, are the main signaling pathways in bacteria, global TCS activity remains poorly described. Here, we report the kinetic parameters of the HK autophosphorylation reaction using previously uncharacterized γ-phosphate-modified ATP analogues to further elucidate their utility as activity-based probes for global TCS analysis. Given the increased stability of thiophosphorylated histidine in comparison to that of the native phosphoryl modification, which is attributed to the decreased electrophilicity of this moiety, we anticipated that ATPγS may be turned over much more slowly by the HKs. Surprisingly, we found this not to be the case, with the turnover numbers decreasing <1 order of magnitude. Instead, we found that alkylation of the thiophosphate had a much more dramatic effect on turnover and, in one case, the binding affinity of this substrate analogue (BODIPY-FL-ATPγS).
中文翻译:

γ-磷酸修饰的ATP类似物对组氨酸激酶自磷酸化作用的探索
尽管由传感器组氨酸激酶(HK)和响应调节剂组成的两组分系统(TCS)是细菌中的主要信号传导途径,但总体TCS活性的描述仍然不多。在这里,我们报告使用以前未表征的γ-磷酸修饰的ATP类似物HK自磷酸化反应的动力学参数,以进一步阐明其作为用于全球TCS分析的基于活性的探针的效用。鉴于硫代磷酸化组氨酸的稳定性与天然磷酸基修饰的稳定性相比有所提高,这归因于该部分的亲电子性降低,我们预期HKs可以更缓慢地转换ATPγS。令人惊讶的是,我们发现情况并非如此,营业额下降了<1个数量级。反而,
更新日期:2018-06-26
中文翻译:

γ-磷酸修饰的ATP类似物对组氨酸激酶自磷酸化作用的探索
尽管由传感器组氨酸激酶(HK)和响应调节剂组成的两组分系统(TCS)是细菌中的主要信号传导途径,但总体TCS活性的描述仍然不多。在这里,我们报告使用以前未表征的γ-磷酸修饰的ATP类似物HK自磷酸化反应的动力学参数,以进一步阐明其作为用于全球TCS分析的基于活性的探针的效用。鉴于硫代磷酸化组氨酸的稳定性与天然磷酸基修饰的稳定性相比有所提高,这归因于该部分的亲电子性降低,我们预期HKs可以更缓慢地转换ATPγS。令人惊讶的是,我们发现情况并非如此,营业额下降了<1个数量级。反而,



























 京公网安备 11010802027423号
京公网安备 11010802027423号