当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting the Unique Mechanism of Bacterial 1-Deoxy-d-xylulose-5-phosphate Synthase
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.biochem.8b00548 David Bartee 1 , Caren L. Freel Meyers 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.biochem.8b00548 David Bartee 1 , Caren L. Freel Meyers 1
Affiliation
|
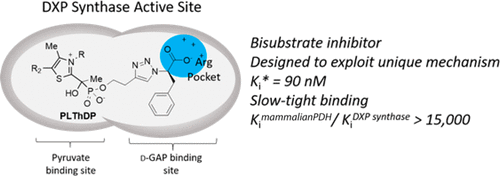
The bacterial metabolite 1-deoxy-d-xyulose 5-phosphate (DXP) is essential in bacterial central metabolism feeding into isoprenoid, thiamin diphosphate (ThDP), and pyridoxal phosphate de novo biosynthesis. Halting its production through the inhibition of DXP synthase is an attractive strategy for the development of novel antibiotics. Recent work has revealed that DXP synthase utilizes a unique random sequential mechanism that requires formation of a ternary complex among pyruvate-derived C2α-lactylthiamin diphosphate (LThDP), d-glyceraldehyde 3-phosphate (d-GAP), and enzyme, setting it apart from all other known ThDP-dependent enzymes. Herein, we describe the development of bisubstrate inhibitors bearing an acetylphosphonate (AP) pyruvate mimic and a distal negative charge mimicking the phosphoryl group of d-GAP, designed to target the unique form of DXP synthase that binds LThDP and d-GAP in a ternary complex. A d-phenylalanine-derived triazole acetylphosphonate (d-PheTrAP) emerged as the most potent inhibitor in this series, displaying slow, tight-binding inhibition with a Ki* of 90 ± 10 nM, forward (k1) and reverse (k2) isomerization rates of 1.1 and 0.14 min–1, respectively, and exquisite selectivity (>15000-fold) for DXP synthase over mammalian pyruvate dehydrogenase. d-PheTrAP is the most potent, selective DXP synthase inhibitor described to date and represents the first inhibitor class designed specifically to exploit the unique E–LThDP–GAP ternary complex in ThDP enzymology.
中文翻译:

针对细菌1-脱氧-d-木酮糖-5-磷酸合酶的独特机制
细菌代谢物1-脱氧d -xyulose -5-磷酸(DXP)是在细菌中心代谢馈送必需成异戊二烯,二磷酸硫胺(THDP),和磷酸吡哆醛从头生物合成。通过抑制DXP合酶来停止其生产是开发新型抗生素的一种有吸引力的策略。最近的工作表明,DXP合酶利用独特的随机顺序机制,该机制要求在丙酮酸衍生的C2α-乙酰硫胺素二磷酸酯(LThDP),d-甘油醛3-磷酸酯(d-GAP)和酶,将其与所有其他已知的ThDP依赖性酶区分开。在本文中,我们描述了双底物抑制剂的开发,该抑制剂具有乙酰基膦酸酯(AP)丙酮酸酯模拟物和模拟d -GAP磷酸基团的远端负电荷,旨在靶向结合LThDP和d -GAP的DXP合酶的独特形式。复杂的。甲d -苯丙氨酸衍生三唑acetylphosphonate(d -PheTrAP)成为在本系列的最有效的抑制剂,显示慢,紧密结合抑制与ķ我* 90±10nM的,向前(的ķ 1)和反向(ķ 2)1.1和0.14分钟的异构化速率分别为–1和DXP合酶相对于哺乳动物丙酮酸脱氢酶的精妙选择性(> 15000倍)。d -PheTrAP是迄今为止描述的最有效,选择性最强的DXP合酶抑制剂,它是专门设计用于开发ThDP酶学中独特的E-LThDP-GAP三元复合物的第一类抑制剂。
更新日期:2018-06-26
中文翻译:

针对细菌1-脱氧-d-木酮糖-5-磷酸合酶的独特机制
细菌代谢物1-脱氧d -xyulose -5-磷酸(DXP)是在细菌中心代谢馈送必需成异戊二烯,二磷酸硫胺(THDP),和磷酸吡哆醛从头生物合成。通过抑制DXP合酶来停止其生产是开发新型抗生素的一种有吸引力的策略。最近的工作表明,DXP合酶利用独特的随机顺序机制,该机制要求在丙酮酸衍生的C2α-乙酰硫胺素二磷酸酯(LThDP),d-甘油醛3-磷酸酯(d-GAP)和酶,将其与所有其他已知的ThDP依赖性酶区分开。在本文中,我们描述了双底物抑制剂的开发,该抑制剂具有乙酰基膦酸酯(AP)丙酮酸酯模拟物和模拟d -GAP磷酸基团的远端负电荷,旨在靶向结合LThDP和d -GAP的DXP合酶的独特形式。复杂的。甲d -苯丙氨酸衍生三唑acetylphosphonate(d -PheTrAP)成为在本系列的最有效的抑制剂,显示慢,紧密结合抑制与ķ我* 90±10nM的,向前(的ķ 1)和反向(ķ 2)1.1和0.14分钟的异构化速率分别为–1和DXP合酶相对于哺乳动物丙酮酸脱氢酶的精妙选择性(> 15000倍)。d -PheTrAP是迄今为止描述的最有效,选择性最强的DXP合酶抑制剂,它是专门设计用于开发ThDP酶学中独特的E-LThDP-GAP三元复合物的第一类抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号