当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic Study of (KCl + N,N-Dimethylformamide + Water) System Based on Potentiometric Measurements
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jced.8b00009 Toba Nasiri-Lohehsara 1 , Bahram Ghalami-Choobar 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jced.8b00009 Toba Nasiri-Lohehsara 1 , Bahram Ghalami-Choobar 1
Affiliation
|
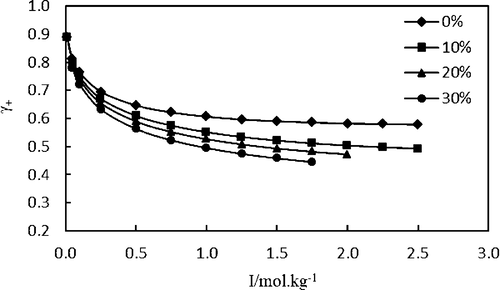
In this research, the thermodynamic properties of (KCl + N,N-dimethylformamide + water) system were determined based on potentiometric technique. The electromotive force measurements were carried out by using self-made electrodes on the galvanic cell of type Ag|AgCl|KCl (m), N,N-dimethylformamide (w), and H2O (1 – w)|K-ISE in various mass fractions of N,N-dimethylformamide in water (0, 0.10, 0.20, and 0.30) in the molality ranging from 0.0094 to 2.5200 mol·kg–1 at T = (298.2 and 308.2) K and P = 0.1 MPa. Thermodynamic properties modeling was implemented using the Debye–Hückel extended equation, Pitzer ion interaction, and Pitzer–Simonson–Clegg models. Subsequently, unknown parameters for each model were determined and utilized to calculate the thermodynamic properties such as the mean activity coefficients, the osmotic coefficients, the excess Gibbs free energy, and the solvent activity for the system under investigation.
中文翻译:

基于电位测量的(KCl + N,N-二甲基甲酰胺+水)体系的热力学研究
在这项研究中,基于电位技术确定了(KCl + N,N-二甲基甲酰胺+水)体系的热力学性质。电动势的测量是使用自制电极在Ag | AgCl | KCl(m),N,N-二甲基甲酰胺(w)和H 2 O(1- w)| K-ISE的原电池上进行的在各种质量分数ñ,ñ二甲基甲酰胺在水(0,0.10,0.20,0.30)在摩尔浓度范围为0.0094至2.5200摩尔公斤· -1在Ť =(298.2和308.2)K和P= 0.1MPa。使用Debye-Hückel扩展方程,Pitzer离子相互作用和Pitzer-Simonson-Clegg模型进行了热力学性质建模。随后,确定每个模型的未知参数,并将其用于计算热力学性质,例如平均活性系数,渗透系数,过量的吉布斯自由能和所研究系统的溶剂活性。
更新日期:2018-06-27
中文翻译:

基于电位测量的(KCl + N,N-二甲基甲酰胺+水)体系的热力学研究
在这项研究中,基于电位技术确定了(KCl + N,N-二甲基甲酰胺+水)体系的热力学性质。电动势的测量是使用自制电极在Ag | AgCl | KCl(m),N,N-二甲基甲酰胺(w)和H 2 O(1- w)| K-ISE的原电池上进行的在各种质量分数ñ,ñ二甲基甲酰胺在水(0,0.10,0.20,0.30)在摩尔浓度范围为0.0094至2.5200摩尔公斤· -1在Ť =(298.2和308.2)K和P= 0.1MPa。使用Debye-Hückel扩展方程,Pitzer离子相互作用和Pitzer-Simonson-Clegg模型进行了热力学性质建模。随后,确定每个模型的未知参数,并将其用于计算热力学性质,例如平均活性系数,渗透系数,过量的吉布斯自由能和所研究系统的溶剂活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号