当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Determination of the CH4 and CO2 Pure Gas Adsorption Isotherms on Different Activated Carbons
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jced.8b00297 Deneb Peredo-Mancilla 1 , Cecile Hort 2 , Mejdi Jeguirim 3 , Camelia Matei Ghimbeu 3 , Lionel Limousy 3 , David Bessieres 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jced.8b00297 Deneb Peredo-Mancilla 1 , Cecile Hort 2 , Mejdi Jeguirim 3 , Camelia Matei Ghimbeu 3 , Lionel Limousy 3 , David Bessieres 1
Affiliation
|
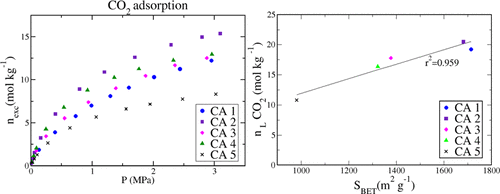
The aim of this work is to provide new experimental data of the adsorption equilibrium of pure CH4 and CO2 on a set of commercial activated carbons and to express it in terms of textural properties such as BET surface area, total pore volume, and micropore volume. Adsorption isotherms up to 3.5 MPa were performed using a homemade manometric technique at 303.15 and 323.15 K. A higher adsorption capacity was found for carbon dioxide than for methane over the whole pressure range for all of the samples. A contribution to the CO2 adsorption capacity of the BET surface area and micropore volume was found. The samples were shown to have a comparable adsorption capacity to that of conventional adsorbents used for the separation and storage of CO2.
中文翻译:

不同活性炭对CH 4和CO 2纯气体吸附等温线的实验测定
这项工作的目的是提供纯CH 4和CO 2在一组商业活性炭上的吸附平衡的新实验数据,并用诸如BET表面积,总孔体积和微孔之类的纹理特性来表达它。体积。使用自制的测压技术在303.15和323.15 K上进行了高达3.5 MPa的吸附等温线。在所有样品的整个压力范围内,二氧化碳的吸附量均高于甲烷。发现对BET表面积和微孔体积的CO 2吸附容量有贡献。样品显示出与常规吸附剂相当的吸附能力,这些常规吸附剂用于分离和储存CO 2。
更新日期:2018-06-27
中文翻译:

不同活性炭对CH 4和CO 2纯气体吸附等温线的实验测定
这项工作的目的是提供纯CH 4和CO 2在一组商业活性炭上的吸附平衡的新实验数据,并用诸如BET表面积,总孔体积和微孔之类的纹理特性来表达它。体积。使用自制的测压技术在303.15和323.15 K上进行了高达3.5 MPa的吸附等温线。在所有样品的整个压力范围内,二氧化碳的吸附量均高于甲烷。发现对BET表面积和微孔体积的CO 2吸附容量有贡献。样品显示出与常规吸附剂相当的吸附能力,这些常规吸附剂用于分离和储存CO 2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号