当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Silver(i)-catalyzed sequential hydroamination and Prins type cyclization for the synthesis of fused benzo-δ-sultams†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8ob00918j B. Maheshwar Rao 1, 2, 3, 4 , J. S. Yadav 1, 2, 3, 4 , B. Sridhar 2, 3, 4, 5 , B. V. Subba Reddy 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8ob00918j B. Maheshwar Rao 1, 2, 3, 4 , J. S. Yadav 1, 2, 3, 4 , B. Sridhar 2, 3, 4, 5 , B. V. Subba Reddy 1, 2, 3, 4
Affiliation
|
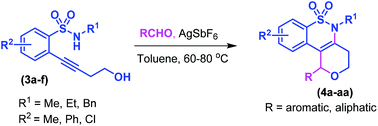
An intramolecular annulation strategy has been developed for the synthesis of tetrahydrobenzo[e]pyrano[4,3-c][1,2]thiazine derivatives by means of coupling of aldehydes with 2-(4-hydroxybut-1-yn-1-yl)-N-arylsulfonamides using a catalytic amount of silver hexafluoroantimonate in toluene at 80 °C. This is the first report on the synthesis of fused benzo-δ-sultam derivatives through C–N, C–O, and C–C bond formations. The reaction proceeds through a cascade of hydroamination and Prins type cyclization.
中文翻译:

银(i)催化的连续加氢胺化和Prins型环化反应,以合成熔融的苯并δ-杜鹃花†
通过醛与2-(4-羟基丁-1-yn-1-偶合)偶联的合成四氢苯并[ e ]吡喃并[4,3- c ] [1,2]噻嗪衍生物的分子内环化策略已经得到发展。在80°C的条件下,使用催化量的六氟锑酸银在甲苯中的溶剂基)生成N-芳基磺酰胺基。这是关于通过C–N,C–O和C–C键的形成合成苯并-δ-sultam衍生物的第一份报告。该反应通过加氢胺化和Prins型环化的级联进行。
更新日期:2018-06-25
中文翻译:

银(i)催化的连续加氢胺化和Prins型环化反应,以合成熔融的苯并δ-杜鹃花†
通过醛与2-(4-羟基丁-1-yn-1-偶合)偶联的合成四氢苯并[ e ]吡喃并[4,3- c ] [1,2]噻嗪衍生物的分子内环化策略已经得到发展。在80°C的条件下,使用催化量的六氟锑酸银在甲苯中的溶剂基)生成N-芳基磺酰胺基。这是关于通过C–N,C–O和C–C键的形成合成苯并-δ-sultam衍生物的第一份报告。该反应通过加氢胺化和Prins型环化的级联进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号