当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The application of a nickel(ii) Schiff base complex in water oxidation: the importance of nanosized materials†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1039/c8cy00582f Hadi Feizi 1, 2, 3, 4 , Farshad Shiri 1, 2, 3, 4 , Robabeh Bagheri 5, 6, 7, 8, 9 , Jitendra Pal Singh 10, 11, 12, 13 , Keun Hwa Chae 10, 11, 12, 13 , Zhenlun Song 5, 6, 7, 8, 9 , Mohammad Mahdi Najafpour 1, 2, 3, 4, 14
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1039/c8cy00582f Hadi Feizi 1, 2, 3, 4 , Farshad Shiri 1, 2, 3, 4 , Robabeh Bagheri 5, 6, 7, 8, 9 , Jitendra Pal Singh 10, 11, 12, 13 , Keun Hwa Chae 10, 11, 12, 13 , Zhenlun Song 5, 6, 7, 8, 9 , Mohammad Mahdi Najafpour 1, 2, 3, 4, 14
Affiliation
|
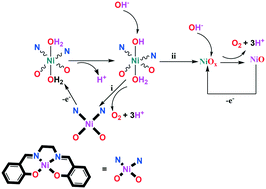
Herein, the role of Ni oxide in the electrocatalytic water oxidation of a nickel(II) Schiff base (N,N′-bis (salicylidene)ethylenediamino nickel(II)) is investigated using scanning electron microscopy, transmission electron microscopy, energy dispersive spectrometry, X-ray diffraction, nuclear magnetic resonance spectroscopy, extended X-ray absorption fine structure, X-ray absorption near edge structure, chronoamperometry, electrochemical methods and theoretical calculations. Although Ni oxide does not have a pure, simple and crystalline structure, the experiments showed that Ni oxide at a pH of 11 is a water-oxidizing catalyst on the surface of a fluorine-doped tin oxide electrode. At pH 3 or 7, no clear water oxidation or NiOx formation was detected. Below this low pH, a solid with high carbon content was detected, which is not a water-oxidizing catalyst. A metal oxide-based mechanism for water oxidation in the presence of a metal complex has not been considered using theoretical calculations. Herein, our theoretical calculations indicated that after the oxidation of the Ni(II) complex, all Ni equatorial bonds in the oxidation state of III were weakened. This effect may cause the decomposition of the intermediate to NiOx. This study could be very promising for the design and synthesis of new, efficient and stable catalysts.
中文翻译:

镍(ii)Schiff碱配合物在水氧化中的应用:纳米材料的重要性†
在此,使用扫描电子显微镜,透射电子显微镜,能量色散光谱法研究了Ni氧化物在镍(II)Schiff碱(N,N'-双(水杨基)乙二氨基镍(II))的电催化水氧化中的作用,X射线衍射,核磁共振波谱,扩展的X射线吸收精细结构,近边缘结构的X射线吸收,计时电流法,电化学方法和理论计算。尽管氧化镍不具有纯净,简单和结晶的结构,但实验表明,pH为11的氧化镍是掺氟氧化锡电极表面的水氧化催化剂。在pH值为3或7时,无清水氧化或NiO检测到x形成。在此低pH值以下,检测到具有高碳含量的固体,该固体不是水氧化催化剂。尚未使用理论计算来考虑在金属络合物存在下基于金属氧化物的水氧化机理。在本文中,我们的理论计算表明,在Ni(II)配合物氧化后,处于III氧化态的所有Ni赤道键均被削弱。这种作用可能导致中间体分解为NiO x。这项研究对于设计,合成新型,高效和稳定的催化剂可能非常有前途。
更新日期:2018-06-26
中文翻译:

镍(ii)Schiff碱配合物在水氧化中的应用:纳米材料的重要性†
在此,使用扫描电子显微镜,透射电子显微镜,能量色散光谱法研究了Ni氧化物在镍(II)Schiff碱(N,N'-双(水杨基)乙二氨基镍(II))的电催化水氧化中的作用,X射线衍射,核磁共振波谱,扩展的X射线吸收精细结构,近边缘结构的X射线吸收,计时电流法,电化学方法和理论计算。尽管氧化镍不具有纯净,简单和结晶的结构,但实验表明,pH为11的氧化镍是掺氟氧化锡电极表面的水氧化催化剂。在pH值为3或7时,无清水氧化或NiO检测到x形成。在此低pH值以下,检测到具有高碳含量的固体,该固体不是水氧化催化剂。尚未使用理论计算来考虑在金属络合物存在下基于金属氧化物的水氧化机理。在本文中,我们的理论计算表明,在Ni(II)配合物氧化后,处于III氧化态的所有Ni赤道键均被削弱。这种作用可能导致中间体分解为NiO x。这项研究对于设计,合成新型,高效和稳定的催化剂可能非常有前途。



























 京公网安备 11010802027423号
京公网安备 11010802027423号