当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
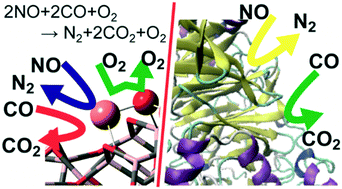
Mechanism of NO–CO reaction over highly dispersed cuprous oxide on γ-alumina catalyst using a metal–support interfacial site in the presence of oxygen: similarities to and differences from biological systems†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8cy00080h Ryoichi Fukuda 1, 2, 3, 4, 5 , Shogo Sakai 1, 2, 3, 4, 5 , Nozomi Takagi 1, 2, 3, 4, 5 , Masafuyu Matsui 1, 2, 3, 4, 5 , Masahiro Ehara 1, 2, 3, 4, 5 , Saburo Hosokawa 1, 2, 3, 4, 5 , Tsunehiro Tanaka 1, 2, 3, 4, 5 , Shigeyoshi Sakaki 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8cy00080h Ryoichi Fukuda 1, 2, 3, 4, 5 , Shogo Sakai 1, 2, 3, 4, 5 , Nozomi Takagi 1, 2, 3, 4, 5 , Masafuyu Matsui 1, 2, 3, 4, 5 , Masahiro Ehara 1, 2, 3, 4, 5 , Saburo Hosokawa 1, 2, 3, 4, 5 , Tsunehiro Tanaka 1, 2, 3, 4, 5 , Shigeyoshi Sakaki 1, 2, 3, 4, 5
Affiliation
|
Copper–alumina (Cu/Al2O3) systems exhibit highly catalytic activity for nitric oxide reduction with carbon monoxide (NO–CO reaction) even in the presence of dioxygen molecule, but the origin of the interesting catalysis remains unclear. Herein, we elucidated the NO–CO reaction mechanism over the Cu/γ-Al2O3 catalyst using DFT and a cluster model consisting of a single Cu2O unit loaded on to a γ-Al2O3 cluster. The DFT calculations showed that the reactions occur via the Cu+/Cu2+ catalytic cycle, which starts from NO dimerization, followed by N2O formation via the first N–O bond scission. The next step is the rate-determining N2O decomposition with the simultaneous formation of a Cu2+ site. The resultant Cu2+ site weakly adsorbs a CO molecule. CO oxidation by surface oxygen occurs with a small activation barrier. On the contrary, CO oxidation with molecular O2 over Cu/γ-Al2O3 is kinetically unfavourable because it needs a large activation energy. The Cu–Al interface plays a crucially important role in NO dimerization and N2O decomposition, which indicates the importance of highly dispersed Cu over Al2O3. Notably, the highly dispersed Cu is not easily oxidized by O2 because O–O bond cleavage is unfavourable compared to N2–O bond cleavage over Cu/γ-Al2O3. This is the origin of the high catalytic activity of the Cu/γ-Al2O3 for the NO–CO reaction even in the presence of O2. The characteristic features of this reaction are similar to NO reduction by nitric oxide/nitrous oxide reductases and CO oxidation by molybdenum CO dehydrogenase from the viewpoint of the key intermediates and electronic processes of the catalytic reaction.
中文翻译:

NO-CO反应的机理上在氧的存在下,使用金属-载体界面部位高度分散在γ一氧化铝催化剂氧化亚铜:相似性,并从生物系统的差异†
铜-氧化铝(Cu / Al 2 O 3)系统即使在存在双氧分子的情况下,也表现出对一氧化碳还原一氧化氮的高催化活性(NO-CO反应),但是有趣的催化作用的起源仍然不清楚。在此,我们阐明了NO-CO的反应机理在铜/γ-Al系2 ö 3使用DFT和由单一的Cu的聚类模型催化剂2加载到的γ-Al O单元2 ö 3簇。DFT计算表明,反应是通过Cu + / Cu 2+催化循环发生的,该循环从NO二聚反应开始,随后形成N 2 O通过第一个N-O键断裂。下一步是确定速率的N 2 O分解,同时形成Cu 2+位点。所得的Cu 2+位点弱吸附CO分子。表面氧导致的CO氧化具有较小的活化势垒。相反,CO氧化用分子直径:2以上的Cu /γ-Al系2 ö 3是动力学上不利的,因为它需要一个大的活化能。Cu-Al界面在NO二聚和N 2 O分解中起着至关重要的作用,这表明高度分散的Cu对Al 2 O 3的重要性。值得注意的是,高度分散的Cu不容易被氧化的ö 2因为相比于N 2 O-O键断裂是不利2 -O键裂解过的Cu /γ-Al系2 ö 3。这是在Cu /γ-Al系的高催化活性的起源2 ø 3,即使在的O-存在下NO-CO反应2。从关键的中间体和催化反应的电子过程的观点来看,该反应的特征类似于由一氧化氮/一氧化二氮还原酶进行的NO还原和由钼CO脱氢酶进行的CO氧化。
更新日期:2018-06-25
中文翻译:

NO-CO反应的机理上在氧的存在下,使用金属-载体界面部位高度分散在γ一氧化铝催化剂氧化亚铜:相似性,并从生物系统的差异†
铜-氧化铝(Cu / Al 2 O 3)系统即使在存在双氧分子的情况下,也表现出对一氧化碳还原一氧化氮的高催化活性(NO-CO反应),但是有趣的催化作用的起源仍然不清楚。在此,我们阐明了NO-CO的反应机理在铜/γ-Al系2 ö 3使用DFT和由单一的Cu的聚类模型催化剂2加载到的γ-Al O单元2 ö 3簇。DFT计算表明,反应是通过Cu + / Cu 2+催化循环发生的,该循环从NO二聚反应开始,随后形成N 2 O通过第一个N-O键断裂。下一步是确定速率的N 2 O分解,同时形成Cu 2+位点。所得的Cu 2+位点弱吸附CO分子。表面氧导致的CO氧化具有较小的活化势垒。相反,CO氧化用分子直径:2以上的Cu /γ-Al系2 ö 3是动力学上不利的,因为它需要一个大的活化能。Cu-Al界面在NO二聚和N 2 O分解中起着至关重要的作用,这表明高度分散的Cu对Al 2 O 3的重要性。值得注意的是,高度分散的Cu不容易被氧化的ö 2因为相比于N 2 O-O键断裂是不利2 -O键裂解过的Cu /γ-Al系2 ö 3。这是在Cu /γ-Al系的高催化活性的起源2 ø 3,即使在的O-存在下NO-CO反应2。从关键的中间体和催化反应的电子过程的观点来看,该反应的特征类似于由一氧化氮/一氧化二氮还原酶进行的NO还原和由钼CO脱氢酶进行的CO氧化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号