当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoselective Tyrosine Bioconjugation through Sulfate Click Reaction
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-07-18 , DOI: 10.1002/chem.201802380 Eun Joung Choi 1 , Dongwook Jung 1 , Jong-Seo Kim 2, 3 , Yan Lee 1 , B. Moon Kim 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-07-18 , DOI: 10.1002/chem.201802380 Eun Joung Choi 1 , Dongwook Jung 1 , Jong-Seo Kim 2, 3 , Yan Lee 1 , B. Moon Kim 1
Affiliation
|
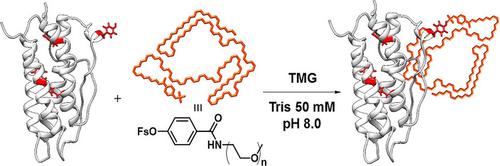
A novel and selective tyrosine functionalization strategy through SuFEx (sulfur fluoride exchange) chemistry is presented. In this approach, free tyrosine (Tyr) reacts selectively with aryl fluorosulfate in the presence of various nucleophilic amino acid residues in bio‐tolerable conditions. Chemoselectivity of this unique SuFEx reaction was confirmed in amino acid, peptide, and protein conjugations. The functions of peptides and proteins were well‐preserved as demonstrated from the Tyr‐specific modification of cell‐penetrating peptide and erythropoietin. This method is well‐suited for residue‐specific modification of native proteins, and thus would expand the versatility of bio‐conjugation in protein chemistry.
中文翻译:

硫酸点击反应的化学选择性酪氨酸生物共轭
通过SuFEx(氟化硫交换)化学,提出了一种新颖的,选择性的酪氨酸官能化策略。在这种方法中,在生物可耐受的条件下,游离酪氨酸(Tyr)在各种亲核氨基酸残基存在下与氟代硫酸芳基酯选择性反应。这种独特的SuFEx反应的化学选择性在氨基酸,肽和蛋白质结合中得到了证实。从穿透细胞的肽和促红细胞生成素的Tyr特异性修饰可以看出,肽和蛋白质的功能得到了很好的保留。此方法非常适合天然蛋白质的残基特异性修饰,因此将扩展蛋白质化学中生物缀合的多功能性。
更新日期:2018-07-18
中文翻译:

硫酸点击反应的化学选择性酪氨酸生物共轭
通过SuFEx(氟化硫交换)化学,提出了一种新颖的,选择性的酪氨酸官能化策略。在这种方法中,在生物可耐受的条件下,游离酪氨酸(Tyr)在各种亲核氨基酸残基存在下与氟代硫酸芳基酯选择性反应。这种独特的SuFEx反应的化学选择性在氨基酸,肽和蛋白质结合中得到了证实。从穿透细胞的肽和促红细胞生成素的Tyr特异性修饰可以看出,肽和蛋白质的功能得到了很好的保留。此方法非常适合天然蛋白质的残基特异性修饰,因此将扩展蛋白质化学中生物缀合的多功能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号