当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
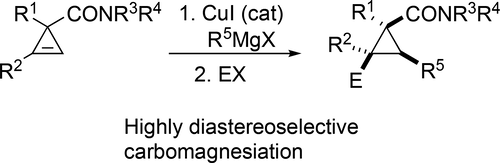
Directed Cu(I)-Catalyzed Carbomagnesiation of 1-Arylcycloprop-2-ene-1-carboxamides En Route to Densely Substituted Functionalized Cyclopropanes
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.joc.8b01063 Andrew Edwards 1 , Michael Rubin 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.joc.8b01063 Andrew Edwards 1 , Michael Rubin 1, 2
Affiliation
|
Copper-catalyzed, directed addition of Grignard reagents across the strained C═C bond of cyclopropene-3-carboxamides was developed. It was demonstrated that the amide functionality serves as an ultimate directing group allowing for highly efficient control of diastereoselectivity of addition including stereoselectivity of electrophilic trapping with prochiral aldehydes. Also, regioselectivity of carbomagnesiation of cyclopropenes with a monosubstituted double bond is investigated. It was shown that in many cases this selectivity is controlled by steric factors and allows for preparation of products with a “reversed” regiochemistry.
中文翻译:

定向Cu(I)催化1-芳基环丙-2-烯-1-羧酰胺的碳还原反应,并转移至重取代的功能化环丙烷中
开发了铜催化的格氏试剂在环丙烯-3-羧酰胺的应变C═C键上的定向加成。已证明酰胺官能团充当最终的导向基团,从而允许高效控制加成的非对映选择性,包括用前手性醛进行亲电捕集的立体选择性。同样,研究了具有单取代双键的环丙烯的碳镁还原的区域选择性。结果表明,在许多情况下,这种选择性受空间因素控制,可以制备具有“逆向”区域化学作用的产物。
更新日期:2018-06-22
中文翻译:

定向Cu(I)催化1-芳基环丙-2-烯-1-羧酰胺的碳还原反应,并转移至重取代的功能化环丙烷中
开发了铜催化的格氏试剂在环丙烯-3-羧酰胺的应变C═C键上的定向加成。已证明酰胺官能团充当最终的导向基团,从而允许高效控制加成的非对映选择性,包括用前手性醛进行亲电捕集的立体选择性。同样,研究了具有单取代双键的环丙烯的碳镁还原的区域选择性。结果表明,在许多情况下,这种选择性受空间因素控制,可以制备具有“逆向”区域化学作用的产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号