当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the mechanisms of aqueous methanol dehydrogenation catalyzed by defined PNP Mn and Re pincer complexes under base-free as well as strong base conditions†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c8cy00746b Zhihong Wei 1, 2, 3 , Adiran de Aguirre 4, 5, 6, 7, 8 , Kathrin Junge 1, 2, 3 , Matthias Beller 1, 2, 3 , Haijun Jiao 1, 2, 3
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c8cy00746b Zhihong Wei 1, 2, 3 , Adiran de Aguirre 4, 5, 6, 7, 8 , Kathrin Junge 1, 2, 3 , Matthias Beller 1, 2, 3 , Haijun Jiao 1, 2, 3
Affiliation
|
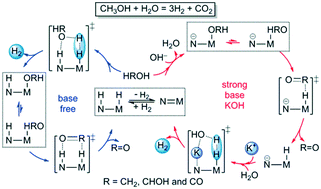
The mechanisms of aqueous methanol dehydrogenation reaction [CH3OH + H2O = 3H2 + CO2] catalyzed by conjugated PNP pincer amido M(CO)2(PNP) and amino HM(CO)2(PNHP) complexes [M = Mn, Re; and PNP = N(CH2CH2P(isopropyl)2)2] under base-free and strong base conditions as well as the K+ promotion effect were studied at the B3PW91 level of density functional theory. Benchmark calculations including dispersion and/or solvation corrections validated the computed gas phase data to be closest to the available kinetic and thermodynamic data from experiments. Under base-free conditions, the innocent mechanism is kinetically more favorable than the non-innocent mechanism. Under strong base conditions, KOH plays a dual role: deprotonating the substrate by OH− and stabilizing the rate-determining transition state by K+ by lowering the free energy barrier for H2 formation by N⋯K+⋯O interaction. Considering the special role of formic acid in H2 storage and CO2 hydrogenation, formic acid dehydrogenation should be accessible under base-free and strong base conditions.
中文翻译:

探索在无碱和强碱条件下,定义的PNP Mn和Re pincer配合物催化的甲醇水溶液脱氢的机理†
PNP夹心酰胺M(CO)2(PNP)和氨基H M(CO)2(PN H P)配合物催化甲醇水溶液脱氢反应[CH 3 OH + H 2 O = 3H 2 + CO 2 ]的机理[M = Mn,Re; 和PNP = N(CH 2 CH 2 P(异丙基)2)2 ]在无碱和强碱条件下以及K +在密度泛函理论的B3PW91级别研究了促进作用。包括色散和/或溶剂化校正在内的基准计算验证了所计算的气相数据最接近于实验中可获得的动力学和热力学数据。在无碱条件下,无害机理在动力学上比非无害机理更有利。下强碱条件下,KOH起着双重作用:去质子被OH基片-和稳定用K速率决定过渡态+通过降低用于h的自由能屏障2由N个⋯ķ形成+ ⋯Ö相互作用。考虑到甲酸在H 2储存和CO 2中的特殊作用 氢化,甲酸脱氢应在无碱和强碱条件下进行。
更新日期:2018-06-22
中文翻译:

探索在无碱和强碱条件下,定义的PNP Mn和Re pincer配合物催化的甲醇水溶液脱氢的机理†
PNP夹心酰胺M(CO)2(PNP)和氨基H M(CO)2(PN H P)配合物催化甲醇水溶液脱氢反应[CH 3 OH + H 2 O = 3H 2 + CO 2 ]的机理[M = Mn,Re; 和PNP = N(CH 2 CH 2 P(异丙基)2)2 ]在无碱和强碱条件下以及K +在密度泛函理论的B3PW91级别研究了促进作用。包括色散和/或溶剂化校正在内的基准计算验证了所计算的气相数据最接近于实验中可获得的动力学和热力学数据。在无碱条件下,无害机理在动力学上比非无害机理更有利。下强碱条件下,KOH起着双重作用:去质子被OH基片-和稳定用K速率决定过渡态+通过降低用于h的自由能屏障2由N个⋯ķ形成+ ⋯Ö相互作用。考虑到甲酸在H 2储存和CO 2中的特殊作用 氢化,甲酸脱氢应在无碱和强碱条件下进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号