当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pseudorotaxanes in the gas phase: structure and energetics of protonated dibenzylamine–crown ether complexes†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c8cp02707b Motoki Kida 1, 2, 3, 4, 5 , Daisuke Shimoyama 1, 2, 3, 4, 5 , Toshiaki Ikeda 1, 2, 3, 4, 5 , Ryo Sekiya 1, 2, 3, 4, 5 , Takeharu Haino 1, 2, 3, 4, 5 , Takayuki Ebata 1, 2, 3, 4, 5 , Christophe Jouvet 6, 7, 8, 9, 10 , Yoshiya Inokuchi 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c8cp02707b Motoki Kida 1, 2, 3, 4, 5 , Daisuke Shimoyama 1, 2, 3, 4, 5 , Toshiaki Ikeda 1, 2, 3, 4, 5 , Ryo Sekiya 1, 2, 3, 4, 5 , Takeharu Haino 1, 2, 3, 4, 5 , Takayuki Ebata 1, 2, 3, 4, 5 , Christophe Jouvet 6, 7, 8, 9, 10 , Yoshiya Inokuchi 1, 2, 3, 4, 5
Affiliation
|
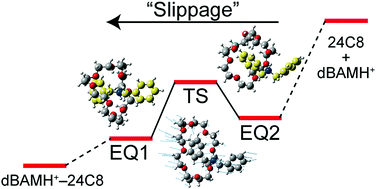
We observe UV spectra of protonated dibenzylamine (dBAMH+) and its complexes with 15-crown-5 (dBAMH+–15C5), 18-crown-6 (dBAMH+–18C6), and 24-crown-8 (dBAMH+–24C8) under cold (∼10 K) gas-phase conditions by UV photodissociation (UVPD) and UV–UV hole-burning (HB) spectroscopy. The UVPD spectrum of the dBAMH+–15C5 complex shows an extensive low-frequency progression, which originates from a unique conformation of the dBAMH+ part with benzene rings facing closely to each other, while UVPD and calculation results suggest open conformations of the dBAMH+ part for dBAMH+–18C6 and dBAMH+–24C8. UV–UV HB spectra of the dBAMH+–24C8 complex indicate that there exist at least two conformers; multiple conformations can contribute to high stability of dBAMH+–24C8 pseudorotaxane due to “conformational” entropic effects. The UVPD experiment indicates that the dissociation probability of dBAMH+–24C8 into dBAMH+ and 24C8 is substantially smaller than that of dBAMH+–15C5 and dBAMH+–18C6, which can be related to the barrier height in the dissociation process. The energetics of the dBAMH+–24C8 complex is investigated experimentally with NMR spectroscopy and theoretically with the global reaction route mapping (GRRM) method. An energy barrier of ∼60 kJ mol−1 is present in the pseudorotaxane formation in solution, whereas there is no barrier in the gas phase. In the course of the photodissociation, excited dBAMH+–24C8 complexes can be trapped at many local minima corresponding to multiple conformations. This can result in effective dissipation of internal energy into degrees of freedom not correlated to the dissociation and decrease the dissociation probability for the dBAMH+–24C8 complex in the gas phase. The energy barrier for the pseudorotaxane formation in solution originates not simply from the slippage process but rather from solvent effects on the dBAMH+–24C8 complex.
中文翻译:

气相中的伪轮烷:质子化的二苄基胺-冠醚配合物的结构和能级†
我们观察了质子化的二苄胺(dBAMH +)及其与15冠5(dBAMH + –15C5),18冠6(dBAMH + –18C6)和24冠8(dBAMH + –24C8 )的配合物的紫外光谱。)在冷(约10 K)气相条件下,通过UV光解离(UVPD)和UV-UV空穴燃烧(HB)光谱。dBAMH + –15C5配合物的UVPD光谱显示出广泛的低频变化,其起因于dBAMH +部分的独特构型,且苯环彼此靠近,而UVPD和计算结果表明dBAMH +的开放构型dBAMH + –18C6和dBAMH +的零件–24C8。dBAMH + –24C8配合物的UV–UV HB光谱表明存在至少两个构象异构体。由于“构象”熵效应,多种构象可有助于dBAMH + –24C8假轮烷的高稳定性。UVPD实验表明,dBAMH + –24C8分解为dBAMH +和24C8的几率显着小于dBAMH + –15C5和dBAMH + –18C6的几率,这与解离过程中的势垒高度有关。dBAMH +的能量–24C8配合物用NMR光谱进行了实验研究,并在理论上采用了全局反应路线图(GRRM)方法进行了研究。溶液中拟轮烷的形成存在约60 kJ mol -1的能垒,而气相中没有能垒。在光离解过程中,激发的dBAMH + –24C8络合物可以被捕获在对应于多种构象的许多局部最小值处。这会导致内部能量有效地耗散到与解离无关的自由度中,并降低dBAMH +的解离概率气相中的–24C8络合物。溶液中假轮烷形成的能垒不仅来自滑移过程,而且还源于溶剂对dBAMH + –24C8配合物的影响。
更新日期:2018-06-22
中文翻译:

气相中的伪轮烷:质子化的二苄基胺-冠醚配合物的结构和能级†
我们观察了质子化的二苄胺(dBAMH +)及其与15冠5(dBAMH + –15C5),18冠6(dBAMH + –18C6)和24冠8(dBAMH + –24C8 )的配合物的紫外光谱。)在冷(约10 K)气相条件下,通过UV光解离(UVPD)和UV-UV空穴燃烧(HB)光谱。dBAMH + –15C5配合物的UVPD光谱显示出广泛的低频变化,其起因于dBAMH +部分的独特构型,且苯环彼此靠近,而UVPD和计算结果表明dBAMH +的开放构型dBAMH + –18C6和dBAMH +的零件–24C8。dBAMH + –24C8配合物的UV–UV HB光谱表明存在至少两个构象异构体。由于“构象”熵效应,多种构象可有助于dBAMH + –24C8假轮烷的高稳定性。UVPD实验表明,dBAMH + –24C8分解为dBAMH +和24C8的几率显着小于dBAMH + –15C5和dBAMH + –18C6的几率,这与解离过程中的势垒高度有关。dBAMH +的能量–24C8配合物用NMR光谱进行了实验研究,并在理论上采用了全局反应路线图(GRRM)方法进行了研究。溶液中拟轮烷的形成存在约60 kJ mol -1的能垒,而气相中没有能垒。在光离解过程中,激发的dBAMH + –24C8络合物可以被捕获在对应于多种构象的许多局部最小值处。这会导致内部能量有效地耗散到与解离无关的自由度中,并降低dBAMH +的解离概率气相中的–24C8络合物。溶液中假轮烷形成的能垒不仅来自滑移过程,而且还源于溶剂对dBAMH + –24C8配合物的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号