Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-06-22 , DOI: 10.1016/j.cplett.2018.06.044 Bahram Ghalami-Choobar , Ali Ghiami-Shomami , Soheila Asadzadeh-Khanghah
|
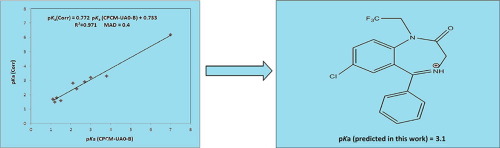
In this study, aqueous acidities of several benzodiazepine (BZD) drugs including Oxazepam, Clonazepam, Nitrazepam, Flunitrazepam, Diazepam, Alprazolam, Estazolam, Temazepam, Medazepam and Bromazepam were successfully computed using two common thermodynamic cycles. To that, calculated gas phase Gibbs free energies at PBE1PBE/6-311+G(d,p) level of theory and computed solvation Gibbs free energies at (CPCM/IEF-PCM: UAKS, UAHF and UA0)/HF/6-31+G(d) levels of theory were combined. The most accurate pKa values were obtained with thermodynamic cycle involving water and using CPCM-UA0 solvation model with MAD = 0.4. Moreover, for the first time, aqueous pKa value of Halazepam was predicted using the best method equal to 3.1.
中文翻译:

某些苯二氮卓类药物的水溶液酸度的第一性原理预测
在这项研究中,使用两个常见的热力学周期成功地计算了几种苯二氮卓(BZD)药物的水溶液酸度,包括奥沙西m,氯硝西am,尼曲西am,氟尼西epa,地西p,阿普唑仑,依他唑仑,替马西m,美达西m和溴马西epa。为此,在理论水平PBE1PBE / 6-311 + G(d,p)处计算出气相吉布斯自由能,在(CPCM / IEF-PCM:UAKS,UAHF和UA0)/ HF / 6-处计算出溶剂化吉布斯自由能。结合了31 + G(d)个理论水平。在涉及水的热力学循环中,使用MAD = 0.4的CPCM-UA0溶剂化模型获得最准确的p K a值。此外,首次使用等于3.1的最佳方法预测了Halazepam的p K a值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号