Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-06-20 , DOI: 10.1016/j.cplett.2018.06.043 Gabriel da Silva
|
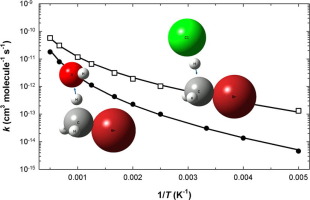
Methyl bromide (CH3Br) is a hazardous ozone depleting substance that is released to the atmosphere from natural and anthropogenic sources. Within the troposphere CH3Br is removed through reaction with •OH and Cl• radicals. Here, quantum and statistical mechanical calculations are combined with existing experimental data in order to arrive at improved rate coefficient expressions for the reaction of methyl bromide with •OH and Cl• from 200 to 2000 K. For CH3Br + •OH k = 9.8×10-14(T/298)2.9exp(-3000/RT) and for CH3Br + Cl•k = 1.5×10-12(T/298)2.0exp(-2900/RT), with k in cm3 molecule-1 s-1, T in K, and R = 1.987 cal mol-1 K-1.
中文翻译:

溴代甲烷与OH和Cl自由基反应的速率系数表达式的改进
甲基溴(CH 3 Br)是一种有害的臭氧消耗物质,会从自然和人为来源释放到大气中。在对流层中,CH 3 Br通过与• OH和Cl •自由基反应而除去。在这里,将量子力学和统计力学计算与现有实验数据结合起来,以得到改进的速率系数表达式,以说明甲基溴与• OH和Cl •在200至2000 K之间的反应。对于CH 3 Br + • OH k = 9.8 ×10 -14(T / 298)2.9曝光(-3000 / RT),对于CH 3 Br + Cl • k = 1.5×10 -12(T / 298)2.0 exp(-2900 / RT),其中k in cm 3分子为-1 s -1,T in K为,R = 1.987 cal mol -1 K -1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号