Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-06-20 , DOI: 10.1016/j.cplett.2018.06.032 Saroj Chowdhury , Prasenjit Mandal , Md. Sarikul Islam , Aslam Hossain , Partha Sarathi Guin , Sanjay Roy , Kalachand Mahali
|
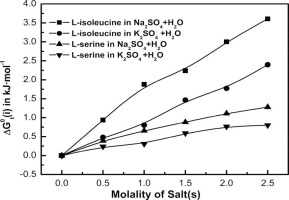
The solubilities of L-isoleucine and L-serine in aqueous sodium sulfate (Na2SO4) and potassium sulfate (K2SO4) from 298.15 to 308.15 K are presented. The solubilities are measured by ‘gravimetric’ method. The relative solubility of the amino acids is calculated to explain the salting-in and salting-out effect in the electrolytes solutions. The amino acids solvation thermodynamical parameters are computed. The chemical effects of the transfer Gibbs energies for both the amino acids are estimated by subtracting the cavity effect and dipole-dipole interaction effect from the total transfer energies. The chemical transfer energetics is employed to discuss solute-solvent and solvent-solvent interactions.
中文翻译:

L-异亮氨酸和L-丝氨酸在Na 2 SO 4和K 2 SO 4水溶液中的溶解度和转移溶剂热动力学从288.15 K到303.15 K
给出了L-异亮氨酸和L-丝氨酸在硫酸钠(Na 2 SO 4)和硫酸钾(K 2 SO 4)中的溶解度为298.15至308.15K。溶解度通过“重量法”测量。计算氨基酸的相对溶解度以解释电解质溶液中的盐析和盐析效果。计算氨基酸溶剂化热力学参数。通过从总转移能中减去空穴效应和偶极-偶极相互作用效应来估算两种氨基酸的转移吉布斯能的化学效应。化学转移能量学被用来讨论溶质-溶剂和溶剂-溶剂的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号