当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arg-Phe-Phe d-Amino Acid Stereochemistry Scan in the Macrocyclic Agouti-Related Protein Antagonist Scaffold c[Pro-Arg-Phe-Phe-Xxx-Ala-Phe-DPro] Results in Unanticipated Melanocortin-1 Receptor Agonist Profiles.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-07-20 , DOI: 10.1021/acschemneuro.8b00218 Mark D Ericson 1 , Zoe M Koerperich 1 , Katie T Freeman 1 , Katlyn A Fleming 1 , Carrie Haskell-Luevano 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-07-20 , DOI: 10.1021/acschemneuro.8b00218 Mark D Ericson 1 , Zoe M Koerperich 1 , Katie T Freeman 1 , Katlyn A Fleming 1 , Carrie Haskell-Luevano 1
Affiliation
|
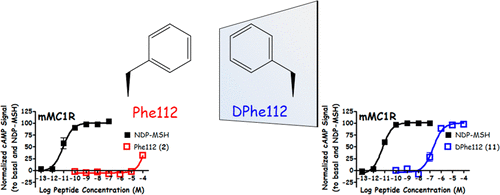
The melanocortin-3 and melanocortin-4 receptors (MC3R and MC4R), endogenous agonists derived from the proopiomelanocortin gene transcript, and naturally occurring antagonists agouti and agouti-related protein (AGRP) have been linked to biological pathways associated with energy homeostasis. The active tripeptide sequence of AGRP, Arg111-Phe112-Phe113, is located on a hypothesized β-hairpin loop. Herein, stereochemical modifications of the Arg-Phe-Phe sequence were examined in the octapeptide AGRP-derived macrocyclic scaffold c[Pro-Arg-Phe-Phe-Xxx-Ala-Phe-DPro], where Xxx was Asn or diaminopropionic acid (Dap). Macrocyclic peptides were synthesized with one, two, or three residues of the Arg-Phe-Phe sequence substituted with the corresponding d-isomer(s), generating a 14 compound library. While l-to-d inversions of the Arg-Phe-Phe sequence in a 20-residue AGRP-derived ligand previously resulted in agonist activity at the MC1R, MC3R, MC4R, and MC5R, only the MC1R was consistently stimulated by the macrocyclic ligands in the present study, with varying ligand potencies and efficacies observed at the MC1R. A general trend of increased MC4R antagonist potency was observed for Dap-containing compounds, while MC5R inverse agonist activity was observed for select ligands. It was observed that stereochemical modification of the Arg-Phe-Phe active tripeptide sequence was insufficient to convert melanocortin antagonist into agonists. Overall, these observations are important in the design of melanocortin ligands possessing potent and selective agonist and antagonist activities.
中文翻译:

大环刺痛相关蛋白拮抗剂支架c [Pro-Arg-Phe-Phe-Xxx-Ala-Phe-DPro]中的Arg-Phe-Phe d-氨基酸立体化学扫描可导致未预期的Melanocortin-1受体激动剂谱。
melanocortin-3和melanocortin-4受体(MC3R和MC4R),源自proopiomelanocortin基因转录物的内源性激动剂以及天然存在的拮抗剂刺豚鼠和刺豚鼠相关蛋白(AGRP)已与与能量稳态相关的生物学途径相关。AGRP的活性三肽序列Arg111-Phe112-Phe113位于假定的β-发夹环上。在此,在八肽AGRP衍生的大环支架c [Pro-Arg-Phe-Phe-Xxx-Ala-Phe-DPro]中检查了Arg-Phe-Phe序列的立体化学修饰,其中Xxx是Asn或二氨基丙酸(Dap )。合成了大环肽,其中的Arg-Phe-Phe序列的一个,两个或三个残基被相应的d-异构体取代,生成了14个化合物文库。虽然在20个残基的AGRP衍生的配体中Arg-Phe-Phe序列从1到3d倒置先前导致了对MC1R,MC3R,MC4R和MC5R的激动剂活性,但只有MC1R始终受到大环配体的刺激在本研究中,在MC1R处观察到了不同的配体效价和功效。对于含有Dap的化合物,观察到了MC4R拮抗剂效能增强的总体趋势,而对于选择的配体,观察到了MC5R反向激动剂活性。观察到,Arg-Phe-Phe活性三肽序列的立体化学修饰不足以将黑皮质素拮抗剂转化为激动剂。总体而言,这些观察对设计具有有效和选择性激动剂和拮抗剂活性的黑皮质素配体很重要。
更新日期:2018-06-20
中文翻译:

大环刺痛相关蛋白拮抗剂支架c [Pro-Arg-Phe-Phe-Xxx-Ala-Phe-DPro]中的Arg-Phe-Phe d-氨基酸立体化学扫描可导致未预期的Melanocortin-1受体激动剂谱。
melanocortin-3和melanocortin-4受体(MC3R和MC4R),源自proopiomelanocortin基因转录物的内源性激动剂以及天然存在的拮抗剂刺豚鼠和刺豚鼠相关蛋白(AGRP)已与与能量稳态相关的生物学途径相关。AGRP的活性三肽序列Arg111-Phe112-Phe113位于假定的β-发夹环上。在此,在八肽AGRP衍生的大环支架c [Pro-Arg-Phe-Phe-Xxx-Ala-Phe-DPro]中检查了Arg-Phe-Phe序列的立体化学修饰,其中Xxx是Asn或二氨基丙酸(Dap )。合成了大环肽,其中的Arg-Phe-Phe序列的一个,两个或三个残基被相应的d-异构体取代,生成了14个化合物文库。虽然在20个残基的AGRP衍生的配体中Arg-Phe-Phe序列从1到3d倒置先前导致了对MC1R,MC3R,MC4R和MC5R的激动剂活性,但只有MC1R始终受到大环配体的刺激在本研究中,在MC1R处观察到了不同的配体效价和功效。对于含有Dap的化合物,观察到了MC4R拮抗剂效能增强的总体趋势,而对于选择的配体,观察到了MC5R反向激动剂活性。观察到,Arg-Phe-Phe活性三肽序列的立体化学修饰不足以将黑皮质素拮抗剂转化为激动剂。总体而言,这些观察对设计具有有效和选择性激动剂和拮抗剂活性的黑皮质素配体很重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号