Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Epigenetic inheritance mediated by coupling of RNAi and histone H3K9 methylation
Nature ( IF 64.8 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0239-3 Ruby Yu 1 , Xiaoyi Wang 1 , Danesh Moazed 1
Nature ( IF 64.8 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0239-3 Ruby Yu 1 , Xiaoyi Wang 1 , Danesh Moazed 1
Affiliation
|
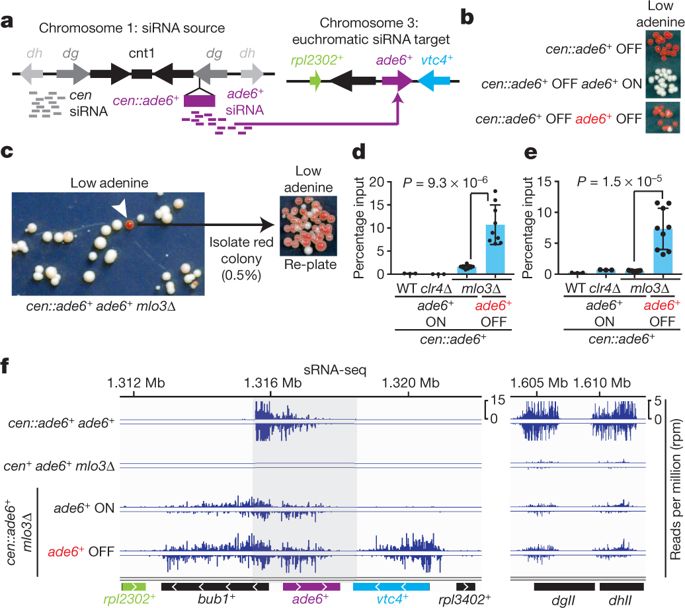
Histone post-translational modifications (PTMs) are associated with epigenetic states that form the basis for cell-type-specific gene expression1,2. Once established, histone PTMs can be maintained by positive feedback involving enzymes that recognize a pre-existing histone modification and catalyse the same modification on newly deposited histones. Recent studies suggest that in wild-type cells, histone PTM-based positive feedback is too weak to mediate epigenetic inheritance in the absence of other inputs3–7. RNA interference (RNAi)-mediated histone H3 lysine 9 methylation (H3K9me) and heterochromatin formation define a potential epigenetic inheritance mechanism in which positive feedback involving short interfering RNA (siRNA) amplification can be directly coupled to histone PTM positive feedback8–14. However, it is not known whether the coupling of these two feedback loops can maintain epigenetic silencing independently of DNA sequence and in the absence of enabling mutations that disrupt genome-wide chromatin structure or transcription15–17. Here, using the fission yeast Schizosaccharomyces pombe, we show that siRNA-induced H3K9me and silencing of a euchromatic gene can be epigenetically inherited in cis during multiple mitotic and meiotic cell divisions in wild-type cells. This inheritance involves the spreading of secondary siRNAs and H3K9me3 to the targeted gene and surrounding areas, and requires both RNAi and H3K9me, suggesting that the siRNA and H3K9me positive-feedback loops act synergistically to maintain silencing. By contrast, when maintained solely by histone PTM positive feedback, silencing is erased by H3K9 demethylation promoted by Epe1, or by interallelic interactions that occur after mating to cells containing an expressed allele even in the absence of Epe1. These findings demonstrate that the RNAi machinery can mediate transgenerational epigenetic inheritance independently of DNA sequence or enabling mutations, and reveal a role for the coupling of the siRNA and H3K9me positive-feedback loops in the protection of epigenetic alleles from erasure.In Schizosaccharomyces pombe, histone H3K9 methylation acts synergistically with short interfering RNA to perpetuate gene silencing during multiple mitotic and meiotic cell divisions.
中文翻译:

RNAi与组蛋白H3K9甲基化偶联介导的表观遗传
组蛋白翻译后修饰 (PTM) 与构成细胞类型特异性基因表达基础的表观遗传状态相关 1,2。一旦建立,组蛋白翻译后修饰可以通过涉及识别预先存在的组蛋白修饰并催化新沉积组蛋白上的相同修饰的酶的正反馈来维持。最近的研究表明,在野生型细胞中,基于组蛋白 PTM 的正反馈太弱,无法在没有其他输入的情况下介导表观遗传 3-7。RNA 干扰 (RNAi) 介导的组蛋白 H3 赖氨酸 9 甲基化 (H3K9me) 和异染色质形成定义了一种潜在的表观遗传机制,其中涉及短干扰 RNA (siRNA) 扩增的正反馈可以直接与组蛋白 PTM 正反馈耦合8-14。然而,目前尚不清楚这两个反馈环的耦合是否可以独立于 DNA 序列以及在没有破坏全基因组染色质结构或转录的突变的情况下维持表观遗传沉默 15-17。在这里,使用裂殖酵母粟酒裂殖酵母,我们表明 siRNA 诱导的 H3K9me 和常染色质基因的沉默可以在野生型细胞的多次有丝分裂和减数分裂细胞分裂过程中以顺式方式进行表观遗传。这种遗传涉及二级 siRNA 和 H3K9me3 向靶基因和周围区域的扩散,并且需要 RNAi 和 H3K9me,这表明 siRNA 和 H3K9me 正反馈环协同作用以维持沉默。相比之下,当仅由组蛋白 PTM 正反馈维持时,沉默会被 Epe1 促进的 H3K9 去甲基化消除,或通过与含有表达等位基因的细胞交配后发生的等位基因间相互作用,即使在没有 Epe1 的情况下也是如此。这些发现表明,RNAi 机制可以独立于 DNA 序列或促成突变介导跨代表观遗传,并揭示了 siRNA 和 H3K9me 正反馈环的耦合在保护表观遗传等位基因免于擦除中的作用。 在粟酒裂殖酵母中,组蛋白H3K9 甲基化与短干扰 RNA 协同作用,在多次有丝分裂和减数分裂细胞分裂期间使基因沉默永久化。
更新日期:2018-06-01
中文翻译:

RNAi与组蛋白H3K9甲基化偶联介导的表观遗传
组蛋白翻译后修饰 (PTM) 与构成细胞类型特异性基因表达基础的表观遗传状态相关 1,2。一旦建立,组蛋白翻译后修饰可以通过涉及识别预先存在的组蛋白修饰并催化新沉积组蛋白上的相同修饰的酶的正反馈来维持。最近的研究表明,在野生型细胞中,基于组蛋白 PTM 的正反馈太弱,无法在没有其他输入的情况下介导表观遗传 3-7。RNA 干扰 (RNAi) 介导的组蛋白 H3 赖氨酸 9 甲基化 (H3K9me) 和异染色质形成定义了一种潜在的表观遗传机制,其中涉及短干扰 RNA (siRNA) 扩增的正反馈可以直接与组蛋白 PTM 正反馈耦合8-14。然而,目前尚不清楚这两个反馈环的耦合是否可以独立于 DNA 序列以及在没有破坏全基因组染色质结构或转录的突变的情况下维持表观遗传沉默 15-17。在这里,使用裂殖酵母粟酒裂殖酵母,我们表明 siRNA 诱导的 H3K9me 和常染色质基因的沉默可以在野生型细胞的多次有丝分裂和减数分裂细胞分裂过程中以顺式方式进行表观遗传。这种遗传涉及二级 siRNA 和 H3K9me3 向靶基因和周围区域的扩散,并且需要 RNAi 和 H3K9me,这表明 siRNA 和 H3K9me 正反馈环协同作用以维持沉默。相比之下,当仅由组蛋白 PTM 正反馈维持时,沉默会被 Epe1 促进的 H3K9 去甲基化消除,或通过与含有表达等位基因的细胞交配后发生的等位基因间相互作用,即使在没有 Epe1 的情况下也是如此。这些发现表明,RNAi 机制可以独立于 DNA 序列或促成突变介导跨代表观遗传,并揭示了 siRNA 和 H3K9me 正反馈环的耦合在保护表观遗传等位基因免于擦除中的作用。 在粟酒裂殖酵母中,组蛋白H3K9 甲基化与短干扰 RNA 协同作用,在多次有丝分裂和减数分裂细胞分裂期间使基因沉默永久化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号