Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure of the adenosine-bound human adenosine A1 receptor–Gi complex
Nature ( IF 64.8 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0236-6 Christopher J Draper-Joyce 1 , Maryam Khoshouei 2, 3 , David M Thal 1 , Yi-Lynn Liang 1 , Anh T N Nguyen 1 , Sebastian G B Furness 1 , Hariprasad Venugopal 4 , Jo-Anne Baltos 1 , Jürgen M Plitzko 2 , Radostin Danev 2 , Wolfgang Baumeister 2 , Lauren T May 1 , Denise Wootten 1, 5 , Patrick M Sexton 1, 5 , Alisa Glukhova 1 , Arthur Christopoulos 1
Nature ( IF 64.8 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0236-6 Christopher J Draper-Joyce 1 , Maryam Khoshouei 2, 3 , David M Thal 1 , Yi-Lynn Liang 1 , Anh T N Nguyen 1 , Sebastian G B Furness 1 , Hariprasad Venugopal 4 , Jo-Anne Baltos 1 , Jürgen M Plitzko 2 , Radostin Danev 2 , Wolfgang Baumeister 2 , Lauren T May 1 , Denise Wootten 1, 5 , Patrick M Sexton 1, 5 , Alisa Glukhova 1 , Arthur Christopoulos 1
Affiliation
|
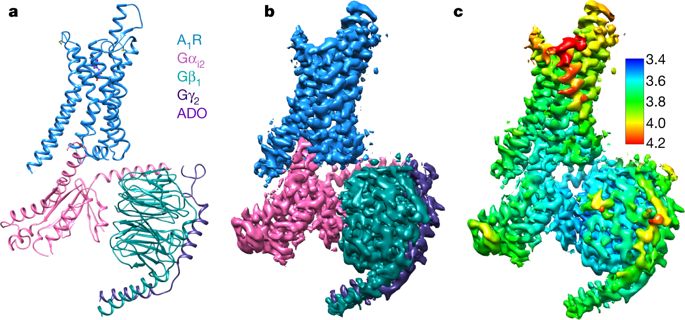
The class A adenosine A1 receptor (A1R) is a G-protein-coupled receptor that preferentially couples to inhibitory Gi/o heterotrimeric G proteins, has been implicated in numerous diseases, yet remains poorly targeted. Here we report the 3.6 Å structure of the human A1R in complex with adenosine and heterotrimeric Gi2 protein determined by Volta phase plate cryo-electron microscopy. Compared to inactive A1R, there is contraction at the extracellular surface in the orthosteric binding site mediated via movement of transmembrane domains 1 and 2. At the intracellular surface, the G protein engages the A1R primarily via amino acids in the C terminus of the Gαi α5-helix, concomitant with a 10.5 Å outward movement of the A1R transmembrane domain 6. Comparison with the agonist-bound β2 adrenergic receptor–Gs-protein complex reveals distinct orientations for each G-protein subtype upon engagement with its receptor. This active A1R structure provides molecular insights into receptor and G-protein selectivity.The cryo-electron microscopy structure of the human adenosine A1 receptor in complex with adenosine and heterotrimeric Gi2 protein provides molecular insights into receptor and G-protein selectivity.
中文翻译:

腺苷结合的人腺苷 A1 受体-Gi 复合物的结构
A 类腺苷 A1 受体 (A1R) 是一种 G 蛋白偶联受体,优先与抑制性 Gi/o 异源三聚体 G 蛋白偶联,与多种疾病有关,但仍然缺乏针对性。在这里,我们报告了由 Volta 相位板冷冻电子显微镜确定的与腺苷和异源三聚体 Gi2 蛋白复合的人类 A1R 的 3.6 Å 结构。与无活性的 A1R 相比,通过跨膜结构域 1 和 2 的运动介导的正构结合位点的细胞外表面收缩。在细胞内表面,G 蛋白主要通过 Gαi α5 C 末端的氨基酸与 A1R 结合-螺旋,伴随着 A1R 跨膜结构域 6 向外移动 10.5 埃。与激动剂结合的 β2 肾上腺素能受体-Gs 蛋白复合物的比较揭示了每个 G 蛋白亚型与其受体结合后的不同方向。这种活性 A1R 结构提供了对受体和 G 蛋白选择性的分子洞察。人类腺苷 A1 受体与腺苷和异源三聚体 Gi2 蛋白复合物的冷冻电子显微镜结构提供了对受体和 G 蛋白选择性的分子洞察。
更新日期:2018-06-01
中文翻译:

腺苷结合的人腺苷 A1 受体-Gi 复合物的结构
A 类腺苷 A1 受体 (A1R) 是一种 G 蛋白偶联受体,优先与抑制性 Gi/o 异源三聚体 G 蛋白偶联,与多种疾病有关,但仍然缺乏针对性。在这里,我们报告了由 Volta 相位板冷冻电子显微镜确定的与腺苷和异源三聚体 Gi2 蛋白复合的人类 A1R 的 3.6 Å 结构。与无活性的 A1R 相比,通过跨膜结构域 1 和 2 的运动介导的正构结合位点的细胞外表面收缩。在细胞内表面,G 蛋白主要通过 Gαi α5 C 末端的氨基酸与 A1R 结合-螺旋,伴随着 A1R 跨膜结构域 6 向外移动 10.5 埃。与激动剂结合的 β2 肾上腺素能受体-Gs 蛋白复合物的比较揭示了每个 G 蛋白亚型与其受体结合后的不同方向。这种活性 A1R 结构提供了对受体和 G 蛋白选择性的分子洞察。人类腺苷 A1 受体与腺苷和异源三聚体 Gi2 蛋白复合物的冷冻电子显微镜结构提供了对受体和 G 蛋白选择性的分子洞察。


























 京公网安备 11010802027423号
京公网安备 11010802027423号