当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
g-Tensor Directions in the Protein Structural Frame of Hyperthermophilic Archaeal Reduced Rieske-Type Ferredoxin Explored by 13C Pulsed Electron Paramagnetic Resonance
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acs.biochem.8b00438 Alexander T. Taguchi 1 , Daijiro Ohmori 2 , Sergei A. Dikanov 3 , Toshio Iwasaki 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acs.biochem.8b00438 Alexander T. Taguchi 1 , Daijiro Ohmori 2 , Sergei A. Dikanov 3 , Toshio Iwasaki 1
Affiliation
|
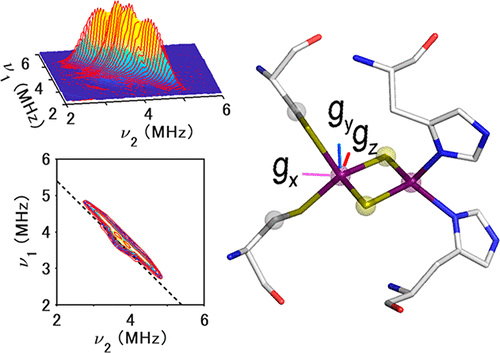
Interpretation of magnetic resonance data in the context of structural and chemical biology requires prior knowledge of the g-tensor directions for paramagnetic metallo-cofactors with respect to the protein structural frame. Access to this information is often limited by the strict requirement of suitable protein crystals for single-crystal electron paramagnetic resonance (EPR) measurements or the reliance on protons (with ambiguous locations in crystal structures) near the paramagnetic metal site. Here we develop a novel pulsed EPR approach with selective 13Cβ-cysteine labeling of model [2Fe-2S] proteins to help bypass these problems. Analysis of the 13Cβ-cysteine hyperfine tensors reproduces the g-tensor of the Pseudomonas putida ISC-like [2Fe-2S] ferredoxin (FdxB). Its application to the hyperthermophilic archaeal Rieske-type [2Fe-2S] ferredoxin (ARF) from Sulfolobus solfataricus, for which the single-crystal EPR approach was not feasible, supports the best-fit gx-, gz-, and gy-tensor directions of the reduced cluster as nearly along Fe–Fe, S–S, and the cluster plane normal, respectively. These approximate principal directions of the reduced ARF g-tensor, explored by 13C pulsed EPR, are less skewed from the cluster molecular axes and are largely consistent with those previously determined by single-crystal EPR for the cytochrome bc1-associated, reduced Rieske [2Fe-2S] center. This suggests the approximate g-tensor directions are conserved across the phylogenetically and functionally divergent Rieske-type [2Fe-2S] proteins.
中文翻译:

通过13 C脉冲电子顺磁共振探索高温嗜热古细菌还原Rieske型铁氧还蛋白的蛋白质结构构架中的g轴方向
在结构和化学生物学的背景下,磁共振数据的解释需要对顺磁性金属辅因子相对于蛋白质结构框架的g-张量方向的先验知识。通常,对于用于单晶电子顺磁共振(EPR)测量的合适蛋白质晶体的严格要求,或对顺磁性金属位点附近质子(晶体结构中位置不明确)的依赖,通常限制了对此类信息的访问。在这里,我们开发了一种新的脉冲与选择性EPR方法13 Ç β模型蛋白质的[2Fe-2S]到帮助旁路这些问题-半胱氨酸标记。在分析13 Ç β -半胱氨酸超精细张量再现克的-tensor恶臭假单胞菌ISC状的[2Fe-2S]铁氧还蛋白(FdxB)。将其应用到Sulfolobus solfataricus的高温嗜热古细菌Rieske型[2Fe-2S]铁氧还蛋白(ARF)中(单晶EPR方法不可行),支持最佳拟合g x-,g z-和g y还原簇的拉伸方向分别接近Fe-Fe,S-S和簇平面法线。简化的ARF g张量的这些近似主方向,由13探索C脉冲EPR从簇分子轴偏斜较小,并且与先前由单晶EPR确定的与细胞色素bc 1相关的还原Rieske [2Fe-2S]中心一致。这表明在整个系统发育上和功能上不同的Rieske型[2Fe-2S]蛋白中,大约的g-张量方向是保守的。
更新日期:2018-06-11
中文翻译:

通过13 C脉冲电子顺磁共振探索高温嗜热古细菌还原Rieske型铁氧还蛋白的蛋白质结构构架中的g轴方向
在结构和化学生物学的背景下,磁共振数据的解释需要对顺磁性金属辅因子相对于蛋白质结构框架的g-张量方向的先验知识。通常,对于用于单晶电子顺磁共振(EPR)测量的合适蛋白质晶体的严格要求,或对顺磁性金属位点附近质子(晶体结构中位置不明确)的依赖,通常限制了对此类信息的访问。在这里,我们开发了一种新的脉冲与选择性EPR方法13 Ç β模型蛋白质的[2Fe-2S]到帮助旁路这些问题-半胱氨酸标记。在分析13 Ç β -半胱氨酸超精细张量再现克的-tensor恶臭假单胞菌ISC状的[2Fe-2S]铁氧还蛋白(FdxB)。将其应用到Sulfolobus solfataricus的高温嗜热古细菌Rieske型[2Fe-2S]铁氧还蛋白(ARF)中(单晶EPR方法不可行),支持最佳拟合g x-,g z-和g y还原簇的拉伸方向分别接近Fe-Fe,S-S和簇平面法线。简化的ARF g张量的这些近似主方向,由13探索C脉冲EPR从簇分子轴偏斜较小,并且与先前由单晶EPR确定的与细胞色素bc 1相关的还原Rieske [2Fe-2S]中心一致。这表明在整个系统发育上和功能上不同的Rieske型[2Fe-2S]蛋白中,大约的g-张量方向是保守的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号