当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hyperconjugation Promotes Catalysis in a Pyridoxal 5′-Phosphate-Dependent Enzyme
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acscatal.8b01911 Steven Dajnowicz 1, 2 , Jerry M. Parks 3 , Xiche Hu 1 , Ryne C. Johnston 3 , Andrey Y. Kovalevsky 2 , Timothy C. Mueser 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acscatal.8b01911 Steven Dajnowicz 1, 2 , Jerry M. Parks 3 , Xiche Hu 1 , Ryne C. Johnston 3 , Andrey Y. Kovalevsky 2 , Timothy C. Mueser 1
Affiliation
|
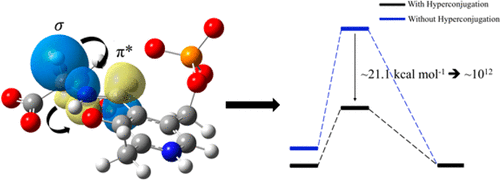
Pyridoxal 5′-phosphate (PLP)-dependent enzymes facilitate reaction specificity by aligning the scissile σ-bond of the PLP-substrate covalent complex perpendicular to the ring of the cofactor. Current models propose that this alignment causes a destabilization of the ground state. To test this hypothesis, quantum chemical calculations, utilizing our recent neutron diffraction models of aspartate aminotransferase, were performed. The calculations reveal that the scissile σ-bond orbital overlaps significantly with the π* orbital of the Schiff base. This σ → π* hyperconjugation interaction stabilizes the ground state of the external aldimine and substantially contributes to transition-state stabilization by withdrawing electron density from the Cα-H σ bond into the π system of PLP, enhancing the rate of catalysis.
中文翻译:

过度共轭促进了吡y醛5'-磷酸盐依赖性酶的催化作用。
吡咯醛5'-磷酸(PLP)依赖性酶可通过使PLP-底物共价复合物的易切割σ键垂直于辅因子环排列来促进反应特异性。当前的模型提出,这种对准导致基态的不稳定。为了验证该假设,利用我们最近的天冬氨酸氨基转移酶的中子衍射模型进行了量子化学计算。计算结果表明,可分裂的σ键轨道与席夫碱的π*轨道显着重叠。这种σ→π*超共轭相互作用可稳定外部醛亚胺的基态,并通过将电子密度从Cα-Hσ键中抽出到PLP的π体系中,从而大大有助于过渡态的稳定化,从而提高了催化速率。
更新日期:2018-06-21
中文翻译:

过度共轭促进了吡y醛5'-磷酸盐依赖性酶的催化作用。
吡咯醛5'-磷酸(PLP)依赖性酶可通过使PLP-底物共价复合物的易切割σ键垂直于辅因子环排列来促进反应特异性。当前的模型提出,这种对准导致基态的不稳定。为了验证该假设,利用我们最近的天冬氨酸氨基转移酶的中子衍射模型进行了量子化学计算。计算结果表明,可分裂的σ键轨道与席夫碱的π*轨道显着重叠。这种σ→π*超共轭相互作用可稳定外部醛亚胺的基态,并通过将电子密度从Cα-Hσ键中抽出到PLP的π体系中,从而大大有助于过渡态的稳定化,从而提高了催化速率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号