当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Directly Bridging Indoles to 3,3′‐Bisindolylmethanes by Using Carboxylic Acids and Hydrosilanes under Mild Conditions
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-08-01 , DOI: 10.1002/asia.201800766 Chang Qiao 1 , Xiao-Fang Liu 1 , Hong-Chen Fu 1 , Hao-Peng Yang 1 , Zhi-Bo Zhang 1 , Liang-Nian He 1, 2
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-08-01 , DOI: 10.1002/asia.201800766 Chang Qiao 1 , Xiao-Fang Liu 1 , Hong-Chen Fu 1 , Hao-Peng Yang 1 , Zhi-Bo Zhang 1 , Liang-Nian He 1, 2
Affiliation
|
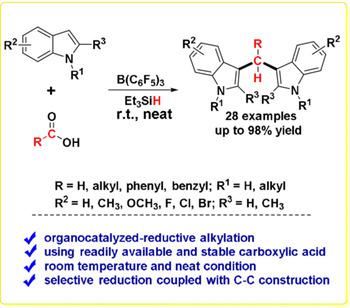
A straightforward Lewis acid‐promoted protocol for 3,3′‐bisindolylmethanes (BIMs) synthesis by reductive alkylation of indoles at the C3 position with carboxylic acids in the presence of hydrosilane was developed for the first time. Instead of aldehydes, more readily available, stable, and easy‐to‐handle carboxylic acids have been employed as alternative alkylating agents. As an efficient organocatalyst, B(C6F5)3 enables the reductive alkylation of various substituted indole derivatives with carboxylic acids with up to 98 % yield at room temperature and under neat conditions. This metal‐free strategy offers an alternative approach for the direct functionalization of indoles to BIMs with carboxylic acids and such protocol allows selective reduction of carboxylic acid to aldehyde in combination with C−C bond formation.
中文翻译:

在温和条件下使用羧酸和氢硅烷直接将吲哚与3,3'-双吲哚甲烷桥联
首次开发了一种简单的路易斯酸促进的3,3'-二吲哚基甲烷(BIM)合成方法,该方法是在存在氢硅烷的情况下,通过羧酸在C3位的吲哚还原烷基化来合成3,3'-二吲哚基甲烷(BIM)。代替醛,更容易获得,稳定和易于处理的羧酸已被用作替代烷基化剂。作为有效的有机催化剂,B(C 6 F 5)3使在室温下和在纯净条件下,各种取代的吲哚衍生物与羧酸进行还原性烷基化反应,收率可达98%。这种无金属的策略为将吲哚直接用羧酸直接官能化为BIM提供了另一种方法,该方案允许将羧酸选择性还原为醛,并形成C-C键。
更新日期:2018-08-01
中文翻译:

在温和条件下使用羧酸和氢硅烷直接将吲哚与3,3'-双吲哚甲烷桥联
首次开发了一种简单的路易斯酸促进的3,3'-二吲哚基甲烷(BIM)合成方法,该方法是在存在氢硅烷的情况下,通过羧酸在C3位的吲哚还原烷基化来合成3,3'-二吲哚基甲烷(BIM)。代替醛,更容易获得,稳定和易于处理的羧酸已被用作替代烷基化剂。作为有效的有机催化剂,B(C 6 F 5)3使在室温下和在纯净条件下,各种取代的吲哚衍生物与羧酸进行还原性烷基化反应,收率可达98%。这种无金属的策略为将吲哚直接用羧酸直接官能化为BIM提供了另一种方法,该方案允许将羧酸选择性还原为醛,并形成C-C键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号