Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-06-20 , DOI: 10.1016/j.bmc.2018.06.026 Hui-ning Li , Hui Wang , Zhi-peng Wang , Hai-ning Yan , Miao Zhang , Yang Liu , Mao-sheng Cheng
|
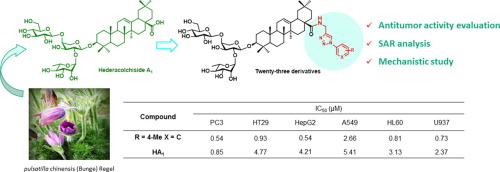
In an attempt to arrive at a more potent antitumor agent than the parent natural saponin hederacolchiside A1, 23 hederacolchiside A1 derivatives (4a-4w) were synthesized via Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition and screened in vitro for cytotoxicity against six human cancer cell lines. The structure-activity relationship of these compounds was elucidated, and the biological screening results showed that most of the compounds exhibited moderate to high levels of antitumor activities against the tested cell lines and some of them displayed more potent inhibitory activities compared with hederacolchiside A1. Compound 4f showed a 2- to 7-fold more potent activity than hederacolchiside A1. The mechanistic study of 4f revealed that this compound can induce cell apoptosis in HepG2 cells via mitochondrial-mediated intrinsic pathways.
中文翻译:

带有芳基三唑部分的新型二十碳五烯皂苷A 1衍生物的合成,抗肿瘤活性评估和机理研究
为了获得比母体天然皂角苷二十碳五烯苷A 1更有效的抗肿瘤剂,通过Cu(I)催化的叠氮化物-炔烃1,3-偶极环加成反应合成了23种二十碳五烯苷A 1衍生物(4a-4w)并进行了筛选。体外对六种人类癌细胞系的细胞毒性。阐明了这些化合物的构效关系,生物学筛选结果表明,大多数化合物对受试细胞系均表现出中等至高水平的抗肿瘤活性,并且其中某些化合物与二十碳五烯苷A 1相比显示出更强的抑制活性。化合物4f表现出的活性比二十碳三烯皂苷A 1高2至7倍。对4f的机理研究表明,该化合物可通过线粒体介导的内在途径诱导HepG2细胞凋亡。


























 京公网安备 11010802027423号
京公网安备 11010802027423号